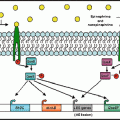
Designation of nerves
Bronchial smooth muscle
Mucociliary system
Bronchial vessels
Parasympathetic nerves1
Constriction
Secretion
Dilation
Sympathetic nerves2
a
Inhibitory nonadrenergic noncholinergic nerves3
Dilation
Excitatory nonadrenergic noncholinergic nerves4
Constriction
Secretion
Dilation
Cough
Increase in permeability
The connection between psychological status and airway health has long been recognized, and over the centuries realization of the need to reduce stress has incorporated in the treatment regime of both infectious and inflammatory respiratory disease, though usually without understanding the underlying biological mechanisms. For instance, in the pre-antibiotic era sanatoria practice, among others, environmental approaches to controlling tuberculosis (TB) involved low stress and emphasis on rest and relaxation techniques. Now, TB is less of a problem to the developed world due to usage of generally effective antibiotics. But, stress is still widely recognized as a predisposing factor to exacerbation of the disease, and re-activation of latent TB infections. In the modern context, asthma is a much more common human respiratory condition, and asthma sufferers worldwide are offered a multitude of supportive treatment methods beyond drugs only, aimed at influencing positively the psychological–endocrinological–immunological feedback loops now viewed as being important in the etiology of the disease.
Is the stress that can exacerbate TB or other respiratory conditions such as asthma causal or reactive in its effects? It is now widely believed that stress-related physiological reactions are contributory to the pathogenesis of a large number of clinical conditions. Importantly, stress may lead not only to a down-regulation of the humoral and cellular immune responses, but depending on the duration of stressor it may also impact the inflammatory threshold (Kemeny and Schedlowski 2007). Perception of stress in human normal and asthmatic subjects was found to positively correlate with the percentage of TNF-alpha producing T cells in the peripheral blood of the asthmatics, but not the control subjects (Joachim et al. 2007). Levels of TNF-alpha were also found to be elevated in chronic obstructive pulmonary disease (Barnes 2008), another condition affected by stress. It is important to understand that an increase in pro-inflammatory cytokines, when localized, is associated with tissue damage, which in itself increases the susceptibility to further injury (which can allergic, infectious, or fibrotic in origin). Unresolved, chronic inflammation predisposes respiratory disease patients to the development of the immunological phenomenon of epitope spreading, in which the adaptive immune response is widened, but paradoxically, remains specific. Such allergen cross-reactivity is thought to play a key role in the overall deterioration of health in patients suffering from asthma (Burastero 2006).
The physiological feedback loops, which are heightened in situations of stress, are the hypothalamus–pituitary–adrenal cortical axis and the sympathetic-adrenal-medullary axis (Chen and Miller 2007). These turn into pathophysiological feedback loops when cognitive and emotional evaluations of a perceived external threat lead to an enhancement of inflammatory response. Cells of the immune system express receptors for catecholamine and glucocorticoid stress hormones, and other factors released during stress, such as neuropeptides (Reiche et al. 2004). Catecholamines and glucocorticoids have been shown to alter secretions or receptor densities of immune cells (Malarkey and Mills 2007). Airway macrophages have an activation profile that differs from other macrophages in the body, and possess receptors for epinephrine to which they respond in a way that increases severity of conditions such as asthma (James and Nijkamp 2000). Neuropeptides can also directly mediate stress-related inflammatory responses because they stimulate mast cells in local proximity to sensory nerve endings (Black 2002). Together, stress released hormones and peptides determine not only the duration of infectious disease, but also the sensitization of the respiratory tract, thereby changing the phenotype of initial airway disease. Stress hormones can also directly exacerbate inflammatory respiratory conditions. When mammals are exposed to repeat stress, alveolar macrophages can become pre-disposed to the development of a more inflammatory phenotype (Broug-Holub et al. 1998). This changes the respiratory milieu, making the organism more likely to show hyperreactivity to stimuli, which can in turn exacerbate the response to respiratory pathogens. Substance P is an example of a neuropeptide the release of which by bronchopulmonary nerves is increased on stress and in addition, is produced by inflammatory cells, which are increased in bronchoalveolar lavage in situations of stress (Joachim et al. 2006).
The lower respiratory tract in several respects represents an immunological entity in its own right because resident immune cells, in particular alveolar macrophages acting as antigen presenting cells and interacting T cells can both influence the type of immune response that favors antibody production, a so-called Th2 immune response. Furthermore, draining lymph nodes do not have a role in dealing with antigen, unlike other parts of the body, excepting immune privileged central nervous tissue. Rather, bronchus-associated lymphoid tissue, so-called BALT and, in the case of more chronic inflammation, tertiary lymphoid organs, assume a more predominant role in the immune response (Constant et al. 2002). Alveolar macrophages also contribute to the epinephrine content of broncho-alveolar lavage (Flierl et al. 2007) and are able to increase the production of epinephrine on activation.
Since stress is such a potent modulator of the immune response and its effector mechanisms (Elenkov et al. 2000), it follows that it may be a contributor to the success of immunization, a mainstay strategy in the prevention of respiratory infections such as influenza and pneumococcal disease. In a recent trial involving young adults receiving the hepatitis B vaccine, those subjects experiencing psychological distress had significantly lower specific antibody responsiveness (Marsland et al. 2006). Drummond and Hewson-Bower (1997) found a correlation between lower serum IgA/albumin ratio and stress in children with recurrent upper respiratory tract infections. Another study concluded that a healthy individual’s increased sociability decreased the risk of viral upper respiratory tract infection (Cohen et al. 2003).
Relatively little is currently known about the impact of immune stressors on transmissibility and infectivity of bacteria colonizing the human respiratory mucosa. A recent murine study demonstrated that repeated stress led to prolonged, induced pathogen carriage, effecting delayed lethality on the first isogenic challenge but impaired protective immunity on second isogenic challenge (Gonzales et al. 2008). The implications of this chain of events for stress in the human work force may be considerable.
10.3 Stress and Its Influence on Susceptibility to Respiratory Infection via Modulation of Respiratory Pathogen Growth and Virulence
While the respiratory tract is usually seen to be in a “low state of alert” vis-à-vis the plethora of aerogenic stimuli (Holt et al. 2008), responding to an infectious organism brings with it the need to do this in a measured and balanced way, in order that the host may regain its steady state integrity, minimizing so-called bystander damage. This is a dual edged sword that the host is wielding and is exemplified by molecules like nitric oxide, which, on the one hand, is part of the respiratory tract’s arsenal to respond to infectious organisms, which on the other hand, under excessive production and conversion to reactive metabolites can lead to tissue damage of the host (van der Vliet et al. 2000). Any stress-related tipping of this fine balance can lead to potentially deleterious local over-inflammation.
At the mucosal interface between host and microorganism, adhesion of bacteria to respiratory epithelial cells is influenced by very different stress-associated factors: bacterial adhesion is improved through expression of proteins that are induced by the paucity of iron which is effected by the host as a means of protection against iron-requiring pathogens (Perez Vidakovics et al. 2007). To the possible detriment of the host, bacterial binding to respiratory epithelia also tends to occur with greater efficiency in the hypersecretory conditions of inflamed airways, when the decoration of mucin glycoconjugates is altered (Davril et al. 1999).
Those microorganisms able to form biofilms are more likely to possess the ability to translocate the respiratory epithelial cell layer into deeper tissues than those that have lesser cohesive and adhesive organization (Yamazakki et al. 2006), and they thereby mediate the progression from inflammation to infection. It is therefore of interest that several of the immune effectors released during stress, particularly the catecholamines norepinephrine, epinephrine and dopamine, can induce changes in the bacterial phenotype relevant to biofilm formation such as adhesion to host epithelia (Vlisidou et al. 2004). In terms of the respiratory tract, catecholamines are naturally present in secretions due the role they play in regulating respiratory function (Table 10.1).
All patient groups share an increased risk of respiratory infection while in hospital; those in intensive care units receiving ventilation are at particular risk of developing so-called ventilation-associated pneumonia (Fridkin 1997). It is known that intubation/long dwelling catheters are associated with biofilm formation and sepsis from bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa (Fridkin 1997). Interestingly, such bacteria have been shown to be receptive to the stress hormones that are elevated in acutely ill patients (Freestone et al. 2008a). What is also of significance is that patients in intensive care are frequently prescribed catecholamine inotropes, drugs that have been shown to increase commensal bacteria biofilm production in intravascular catheters (Lyte et al. 2003), as well as resuscitation of antibiotic-damaged pathogenic bacteria (Freestone et al. 2008b).
How might the catecholamines that are released into the respiratory mucosa during acute stress, or applied therapeutically, influence the infectivity of any potentially pathogenic bacteria within the vicinity of the elaborated hormone? The first context is growth. All pathogenic bacteria require iron to grow in vivo. This growth is prevented due to the presence of lactoferrin and transferrin, key innate immune defense proteins the role of which is to bind all free ferric iron, thereby making blood and respiratory secretions too low in iron to support the growth of iron-requiring pathogens. To overcome this usually very effective iron-limitation defense of host fluids, bacteria produce siderophores, very high affinity secreted ferric iron binding molecules, which allow them to acquire the transferrin and lactoferrin-sequestered iron. Work from several groups has shown that catecholamine stress hormones can also function as direct bacterial siderophores (Freestone et al. 2000, 2002) (see also Chap. 3 ). This stress hormone provision of iron can induce up to a million fold increase in the growth of pathogenic bacteria, including those causing airway infections such as Klebsiella pneumoniae and Pseudomonas aeruginosa and Bordetella species (Freestone et al. 1999; Alverdy et al. 2000; Anderson and Armstrong 2006, 2008). Epinephrine, norepinephrine, dopamine, and their metabolites can all reduce the iron-limiting function of bacteriostatic, key innate defense, proteins lactoferrin, and transferrin (Freestone et al. 2002). Through interaction of these host iron-binding proteins with stress hormones, blood and epithelial secretions are rendered much less bacteriostatic as bacteria are able to access normally inaccessible host sequestered iron sources (Alverdy et al. 2000; Freestone et al. 2000, 2002, 2008a; Anderson and Armstrong 2006, 2008). Bordetella species, significant respiratory pathogens of infants (causing whooping cough), also make use of stress hormones to increase transcription of several iron acquisition components (Anderson and Armstrong 2006, 2008). Dopamine, norepinephrine, and epinephrine are all able to induce the transcription of bfeA, the gene for the Bordetella enterobactin/catechol xenosiderophore receptor. In addition, as has been shown for many enteric and skin bacteria (Freestone et al. 1999), norepinephrine also stimulate growth of Bordetella bronchiseptica growth in iron-limiting medium containing serum, via enabling the bacteria to access transferrin bound iron (Anderson and Armstrong 2006, 2008). As already noted, respiratory secretions are markedly iron limited, though the action of host iron binding proteins, and catecholamines may therefore represent a route by which respiratory pathogens, such as B. bronchiseptica, could obtain essential iron in the host environment (Anderson and Armstrong 2006, 2008). Exposure of respiratory pathogens to stress-released hormones can also induce the bacteria to synthesize their own growth factors, the mechanism of action of which is non-transferrin dependent (Freestone et al. 1999). Thus, the effects of stress hormone exposure on bacteria can be manifest long after the initial stress event has ended, and catecholamine levels returned to normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree