Fig. 15.1
Characteristic features of immature and mature dendritic cells (DCs)
Generally, cancer antigens or the long peptides are engulfed by DCs, digested into helper and killer epitope peptides by the cellular enzymes such as cathepsins in lysosome, transported to the cell surface by exocytosis, and presented to T cell receptors (TCRs) on CD4+ T cells through MHC class II to induce effector T helper (Th) cells. On the other hand, endogenous antigens are degraded by proteasome and transported to the cell surface to present to CD8+ T cells through MHC class I to induce cytotoxic killer T (Tc) cells. Some antigens engulfed by DCs are digested in lysosome to give killer epitopes. The killer epitope peptides are then transported to endoplasmic reticulum (ER) and bind to MHC class I presented to CD8+ T cells, so-called ‘cross presentation’. Antigen-specific Th cells producing cytokines such as IL-2 and IFN-γ encourages the induction of Tc cells (Fig. 15.2).
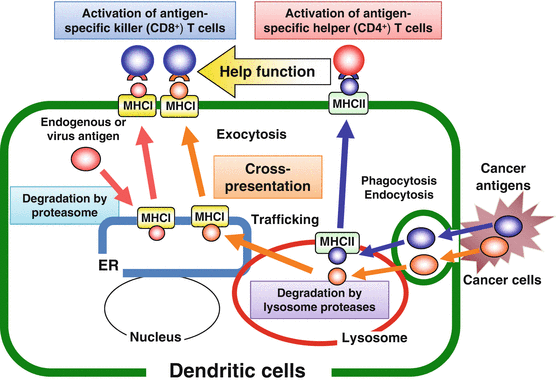

Fig. 15.2
Antigen presentation pathways in dendritic cells (DCs)
15.2 Regulation of Antigen-Presenting Function of DCs
DCs, representative APCs, effectively induce anti-tumor immune responses through activation of antigen-specific CD4+ and CD8+ T cells. In tumor microenvironments, DCs engulf cancer-derived antigens and generate Th and Tc cells in the draining lymph node. The induced effector Tc cells recognize and kill the target cancer cells in response to the antigens. In vaccination, long peptide-pulsed DCs strongly prime both CD4+ and CD8+ T cells capable of recognizing and killing tumor cells in response to the antigens on the target cells. Therefore, controlling the DC activation and the subsequent effective antigen presentation is required for application of DC-mediated cancer immunotherapy. In this chapter, we report on the regulating mechanisms of the antigen-presenting function of DCs mediated by TLR ligands, type 1 cytokines, and CD40/40L interaction and the possibility of their application in cancer immunotherapy.
15.2.1 TICAM-1 (TRIF) Signaling in DCs Regulates Major Histocompatibility Complex (MHC) Class II Expression and CD4+ T Cell Activation
Stimulation with TLR ligands such as LPS strongly upregulates surface MHC class II expression levels on DCs, which play a central role in immune responses by presenting antigenic peptides to CD4+ T cells. It has been reported that surface expression of MHC class II is mainly regulated by the post-transcriptional mechanism in DCs. TICAM-1 [TRIF (TIR-domain-containing adapter-inducing IFN-β)]-, but not the MyD88-, dependent pathway of LPS signaling in DCs is crucial for the surface expression of MHC class II, followed by CD4+ T cell activation. LPS enhanced RhoB activity, but not RhoA, Cdc42, or Rac1/2 in a dependent manner. Transduction of dominant-negative (DN) form of the RhoB gene or small-interfering RNA (siRNA) against RhoB significantly blocked the LPS-induced surface expression of MHC class II on DCs. In addition, GEFH1 associated with RhoB and DN-GEFH1 or siRNA of GEFH1 suppressed the LPS-mediated RhoB activation and surface expression of MHC class II. Furthermore, DN-RhoB attenuated the antigen-presenting function of DCs against CD4+ T cells. Therefore, these results not only provide a molecular mechanism relating to how the surface expression of MHC class II is regulated during the maturation of DCs, but also suggest that the activation of the TICAM-1 –GEFH1–RhoB pathway in DCs might be a promising target for controlling the activation of antigen-specific CD4+ T cells in cancer immunotherapy (Kamon et al. 2006).
15.2.2 Zinc Homeostasis Is Involved in DC Maturation
Zinc, a trace element, is required for the function of many enzymes and transcription factors, controlling cell growth, development, and differentiation. Generally, lack of zinc causes defects in innate and acquired immune responses. In DCs, stimulation with LPS, the TLR4 agonist, induced the expression of zinc exporters (Znt family) and reduced zinc importers (Zip family), and thereby decreased intracellular free zinc. The LPS-induced alterations in zinc transporter expression were dependent on TICAM-1 (TRIF) but not MyD88. TPEN, a zinc chelator, mimicked the effects of LPS, whereas zinc supplementation with pyrithione in DCs or transduction of the Zip6 gene, a zinc transporter whose expression was reduced by LPS, inhibited LPS-induced surface expression of MHC class II and co-stimulatory molecules. In addition, TPEN-treated DCs were capable of activating antigen-specific CD4+ T cells. In the presence of antigen peptide, TPEN- or LPS-treated DCs induced augmented IL-2 production by CD4+ T cells compared with untreated DCs. Thus, LPS-induced reduction of intracellular free zinc levels is a critical step in the antigen-presenting function of DCs (Fig. 15.3). These results suggest a correlation between TLR–TICAM-1 (TRIF) signaling cascade and transporter-mediated intracellular zinc homeostasis in DC maturation (Kitamura et al. 2006).
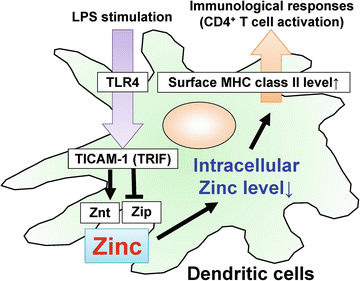

Fig. 15.3
TICAM-1 [TRIF (TIR-domain-containing adapter-inducing interferon-β)]-dependent alteration of zinc transporters in dendritic cells (DCs) controls antigen presentation to CD4+ T cells
15.2.3 Neuropeptide Signaling in DCs Activates Type-1 Immune Responses
Substance P (SP) and neurokinin (NK) A, both neurotransmitters, are widely distributed in both the central and peripheral nervous system. Recently, it was demonstrated that IFN-γ remarkably induced NK-2 receptor (NK2R) expression of DCs. This finding strongly suggests that neuropeptide signaling may be closely related to regulation of DC-mediated immune responses. The effect of neuropeptide signaling on function of DCs was then investigated. DCs treated with IFN-γ or LPS remarkably induced the NK2R gene in a STAT (signal transducer and activator of transcription) 1-dependent manner. LPS-induced NK2R gene expression was dependent on IFNAR1, suggesting that type 1 IFNs induced by LPS were critical in the induction of the NK2R gene. Moreover, it was confirmed that surface NK2R expression and NKA production levels were significantly elevated after IFN-γ or LPS stimulation. Transduction of the NK2R gene into DCs augmented the expression level of surface MHC class II and promoted antigen-specific IL-2 production by CD4+ T cells. Furthermore, blockade of NK2R by an antagonist significantly suppressed IL-2 and IFN-γ production by CD4+ or CD8+ T cells after stimulation with the antigen-loaded DCs. Finally, it was confirmed that human DCs also enhanced expression levels of the NK2R and TAC-1 genes, encoding both SP and NKA, after the IFN-γ or poly I:C stimulation (Fig. 15.4). Thus, these findings indicate that NK2R-dependent neuropeptide signaling regulates type 1 immunity through the activation of DC function including antigen presentation to effector T cells (Kitamura et al. 2012).
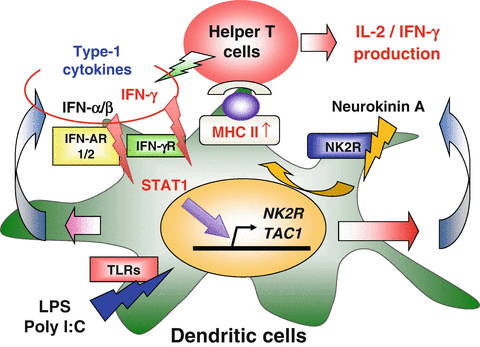

Fig. 15.4
Neurokinin-2 receptor (NK2R)-dependent neuropeptide signaling activates dendritic cell (DC) function in type 1 immune responses
15.2.4 CD40/40L-Mediated Cell-to-Cell Interaction Augments DC Function
In vivo injection with α-galactosylceramide (α-GalCer), the ligand for natural killer T (NKT) cells shows strong anti-tumor activities. Because these activitieswere similar to those of IL-12, it was thought that the involvement of IL-12 in the activation of NKT cells was caused by α-GalCer. In fact, production of IFN-γ by NKT cells in response to α-GalCer was required for IL-12 produced by DCs and direct interaction between NKT cells and DCs through CD40/CD40L. The combination therapy using suboptimal doses of α-GalCer together with suboptimal doses of IL-12 synergistically enhanced natural killing activity and IFN-γ production. These findings indicate an important role of CD40/40L interaction for inducing IL-12 production by DCs in the activation of NKT cells by α-GalCer. Therefore, the antigen-loaded NKT cells may be able to activate DCs for subsequent immune responses, suggesting a promising strategy for immunotherapy of cancers (Kitamura et al. 1999).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree