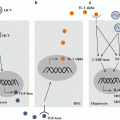
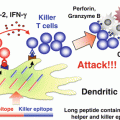
Fig. 13.1
Invariant natural killer T (iNKT) cell-mediated adjuvant effects on T cells are dependent on the innate immunity. Intravenously administered artificial adjuvant vector cells (aAVCs) are killed by iNKT and natural killer (NK) cells (upper, right), and cellular debris is captured by endogenous dendritic cells (DCs) (right, middle). α-Galactosylceramide (α-GalCer) on the aAVCs activates iNKT cells, which secrete cytokines that lead to DC maturation (right, middle). Mature DCs are then able to induce an antigen-specific T cell response (right, lower). They also cross-present glycolipid from phagocytosed aAVCs to iNKT cells in a CD1d-dependent manner
We then demonstrated that human cells transfected with ovalbumin (OVA) antigen and loaded with α-GalCer could also generate both innate and adaptive immunity in wild-type mice. We also assessed the safety of this approach in a canine large animal model. Physical examination, blood chemistry analysis, and autoantibody tests, as well as tissue biopsies from liver, lung, and other organs from dogs given high-dose aAVCs, confirmed the safety of this method in addition to its efficacy in generating immunologic responses (Shimizu et al. 2013).
A final preclinical study was performed to determine whether tumor antigen-specific T cell responses seen in allogeneic studies could also be generated in an autologous setting. For this purpose, we developed a novel humanized mouse model using NOD/Shi-scid/IL-2Rγc null (NOG) mice. T cells from healthy HLA-A2+ donors were transduced with the MART (melanoma antigen recognized by T cells)-1 tumor antigen TCR gene and adoptively transferred into NOG mice. This was followed by intravenous transfer of syngeneic immature DCs and iNKT cells. Finally, aAVCs transduced with MART-1 mRNA and loaded with α-GalCer were transferred into one group of mice but not controls. As expected, the transferred TCR gene-transduced T cells expanded robustly in mice receiving MART-1 expressing aAVC but not controls (Shimizu et al. 2013).
Thus far, our current pre-clinical studies demonstrate the safety and efficacy of aAVCs in harnessing the innate and adaptive immune systems through targeting of in situ DCs. This model system, which uses different arms of the immune system to generate a systemic and specific anti-tumor response, could prove clinically beneficial as immunotherapies against a number of malignant diseases.
References
Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV (2005) Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med 201(9):1503–1517CrossRefPubMedCentralPubMed
Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, Yamamura T, McMahon-Pratt D (2008) Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol 38(3):706–719CrossRefPubMedCentralPubMed
Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM (2003) Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a co-administered protein. J Exp Med 198(2):267–279CrossRefPubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree