Fig. 14.1
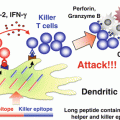
Strategy of Wilms’ tumor gene (WT1) product-derived peptide vaccination combined with bacillus Calmette-Guérin cell wall skeleton (BCG-CWS). Intradermally (i.d.) injected BCG-CWS binds to Toll-like receptor (TLR) 2 and 4 on dendritic cells (DCs), and activates immature DCs into mature form. Major histocompatibility complex (MHC) class I, CD80, and CD86, on matured DCs are upregulated. And matured DCs produce interleukin (IL)-12. Under this condition, i.d. injected WT1 peptide binds to upregulated MHC class I molecules on matured DCs, and efficiently induces WT1-specific cytotoxic T lymphocytes (CTLs)
In the WT1 peptide vaccination combined with BCG-CWS study (Nakajima et al. 2004), WT1 peptide was injected 1 day after injection of BCG-CWS for the following reasons: (1) it takes some time for BCG-CWS to activate DCs from immature to mature forms that have higher potency to induce antigen-specific immune responses; and (2) the activated DCs are required to stay at the site of BCG-CWS injection until the injection of WT1 peptide. In fact, immunization with a mixture of BCG-CWS and WT1 peptide did not provide better effects on tumor rejection and growth inhibition than immunization with WT1 peptide alone in our preliminary studies (data not shown).
14.2.2 BCG-CWS Enhances Potency of WT1 Peptide Vaccination
14.2.2.1 Effect of WT1 Peptide Vaccination Combined with BCG-CWS on Rejection of Implanted Tumors
To investigate the in vivo effect of vaccination with WT1 peptide combined with BCG-CWS, mice were implanted with WT1-expressing leukemia cells (mWT1-C1498) and treated with the WT1 peptide vaccination combined with BCG-CWS. The tumor implantation and vaccination schedules are shown in Fig. 14.2. On day 0, mice were intraperitoneally (i.p.) implanted with 5 × 105 mWT1-C1498 tumor cells in 100 μl of phosphate buffered saline (PBS). On day 1, 100 μg of BCG-CWS was i.d. injected in the abdomen, followed by i.d. injection of 100 μg of WT1 peptide [MHC class I (H-2Db)-binding peptide, Db126, a.a. 126–134 RMFPNAPYL] at the same site as that of BCG-CWS injection on day 2. This treatment was repeated four times at weekly intervals. In control groups, either WT1 peptide, BCG-CWS, or PBS was injected on days 1, 8, 15, and 22. Tumor growth was monitored by measuring the longest diameter of the palpable mass.
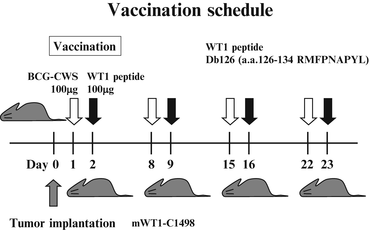

Fig. 14.2
In vivo tumor challenges and vaccination schedule. Mice were intraperitoneally (i.p.) implanted with 5 × 105 mWT1-C1498 cells on day 0. In the bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) and Wilms’ tumor gene (WT1) product-derived peptide group, 100 μg of BCG-CWS was intradermally (i.d.) injected in the abdomen on days 1, 8, 15, and 22, followed by i.d. injection of 100 μg of WT1 peptide at the same site as that of BCG-CWS injection on days 2, 9, 16, and 23. In control groups, either 100 μg of BCG-CWS, 100 μg of WT1 peptide, or phosphate buffered saline (PBS) was i.d. injected in the abdomen on days 1, 8, 15, and 22
Four of the 13 BCG-CWS- and WT1 peptide-treated mice rejected implanted tumor and survived until day 65. On the other hand, none of the BCG-CWS-treated, WT1 peptide-treated, and non-treated mice did not reject implanted tumors (Fig. 14.3a). Disease-free survival of BCG-CWS- and WT1 peptide-treated mice was 31 % and was significantly higher than that of the other three groups (p < 0.05) (Fig. 14.3b). WT1 peptide vaccination combined with BCG-CWS effectively rejected implanted tumors.
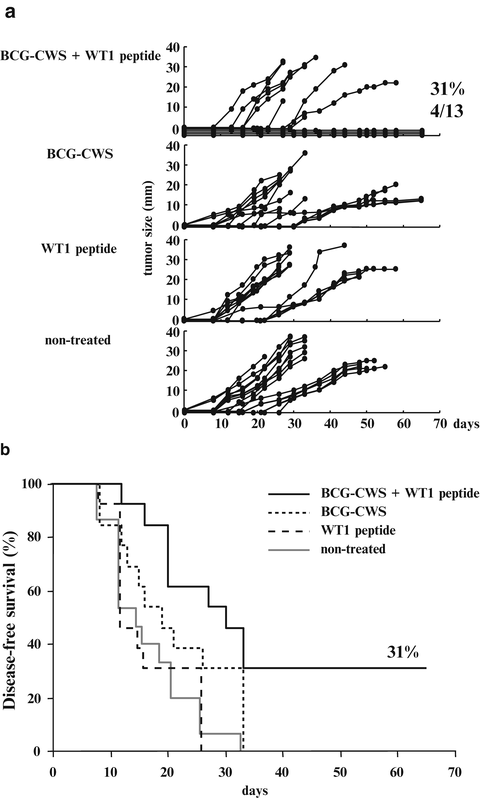

Fig. 14.3
Effect of Wilms’ tumor gene (WT1) product-derived peptide vaccination combined with bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) on rejection of implanted WT1-expressing tumor cells. (a) The tumor growth curves of the four groups. Each line represents the tumor size of individual mice. Tumor sizes represent the longest diameters. (b) Disease-free survival curves of the four groups. Solid black, dotted, broken, and solid gray lines represent disease-free survival curves of mice treated with BCG-CWS and WT1 peptide, BCG-CWS alone, WT1 peptide alone, and non-treated mice, respectively
14.2.2.2 Induction of WT1-Specific Cytotoxic T Lymphocytes (CTLs) by Immunization with BCG-CWS and WT1 Peptide
Splenocytes from the mice treated four times with BCG-CWS and WT1 peptide or from non-treated mice were in vitro re-stimulated with WT1 peptide and assayed for cytotoxic activity against WT1-expressing mWT1-C1498 and WT1-nonexpressing C1498 cells (Fig. 14.4). The splenocytes from the immunized mice showed a significant WT1-specific lysis against WT1-expressing mWT1-C1498 compared with that against WT1-non-expressing C1498. In contrast, the splenocytes from the non-treated mice did not show a significant WT1-specific lysis against the target cells. These results demonstrated that WT1 peptide and BCG-CWS treatment could induce WT1-specific CTL responses, resulting in suppression of the growth of the implanted tumor cells.
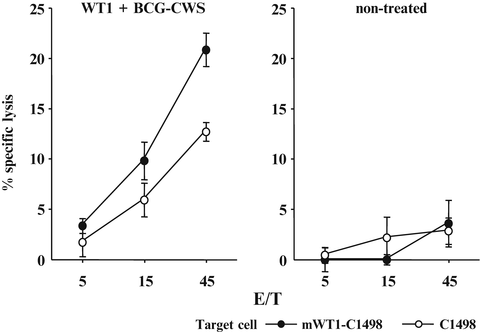

Fig. 14.4
Induction of Wilms’ tumor gene (WT1) product-derived peptide-specific cytotoxic T lymphocytes (CTLs) by immunization with bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) and WT1 peptide. Splenocytes from the mice immunized four times with BCG-CWS and WT1 peptide or from non-treated mice were in vitro re-stimulated with WT1 peptide-pulsed synergistic splenocytes. Their cytotoxic activities against WT1-expressing mWT1-C1498 or WT1-non-expressing C1498 were tested by 51Cr-release cytotoxicity assay at the indicated effector cell to target cell (E/T) ratios in triplicate. Closed and open circles represent cytotoxic activities against mWT1-C1498 and C1498 cells, respectively. Bars indicate standard errors
14.3 Type I Interferon (IFN): IFN-β
14.3.1 Strategy of Co-administration of WT1 Peptide Vaccine and IFN-β
Interferon (IFN)-β is a type I interferon, and is known for its various anticancer properties: (1) enhancement of the expression of many surface molecules that are essential for binding and/or activation of CTLs, in particular the MHC class I as well as the receptors B7-1 (CD80) and intercellular adhesion molecule-1 (ICAM-1) (Dhib-Jalbut and Cowan 1993; Dezfouli et al. 2003), on antigen-presenting cells (APCs) or cancer cells; (2) activation of natural killer (NK), B, and T cells (Kayagaki et al. 1999; Sato et al. 2001; Kirkwood et al. 2002); (3) a direct anti-proliferation effect on cancer cells by promoting cell cycle arrest at the G1 phase (Tanabe et al. 2000); (4) induction of apoptosis of cancer cells (Chen et al. 2001); and (5) inhibition of angiogenesis (Streck et al. 2004). In fact, it was reported in mouse models that type I IFN was essential in the induction and augmentation of CTLs (Wakita et al. 2006; Gehring et al. 2005). Furthermore, type I IFN, IFN-β, and/or IFN-α has already been in use for cancer immunotherapy in clinical settings (Kirkwood et al. 2002; Mani et al. 1996; Fine et al. 1997; Beppu et al. 2003; Watanabe et al. 2005; Gresser 2007; Anguille et al. 2011), and the mechanism for the enhancement of immunity against cancer has been thoroughly investigated (Dhib-Jalbut and Cowan 1993; Dezfouli et al. 2003; Kayagaki et al. 1999; Sato et al. 2001; Kirkwood et al. 2002; Tanabe et al. 2000; Chen et al. 2001; Streck et al. 2004). Considering these reports, IFN-β should be considered as one of the most promising immunopotentiating agents for use with TAA-directed cancer vaccines.
We focused on the immunopotentiating ability of IFN-β, and planed the co-administration of WT1 peptide vaccine and IFN-β (Nakajima et al. 2012). IFN-β increases MHC class I molecules on tumor cells, and activates T cells and NK cells (Kayagaki et al. 1999; Sato et al. 2001; Kirkwood et al. 2002). Activated NK cells produce IFN-γ, which in turn activates DCs, T cells, and NK cells (Degli-Esposti and Smyth 2005; Fedele et al. 2004; He et al. 2007). Under this condition, injected WT1 peptide vaccine induces WT1-specific CTLs efficiently. Furthermore, these reactions gave CTLs an advantage in killing MHC high-expressing tumor cells (Dhib-Jalbut and Cowan 1993; Dezfouli et al. 2003). On the other hand, IFN-β-activated NK cells kill the remaining MHC non- or low-expressing tumor cells (Fig. 14.5).
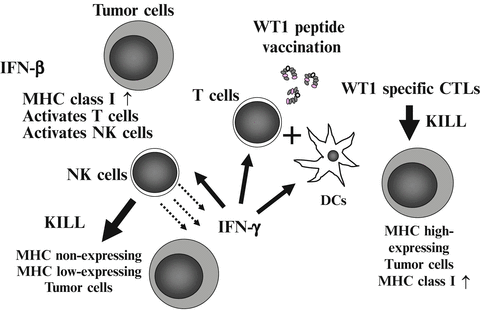

Fig. 14.5
Strategy of co-administration of Wilms’ tumor gene (WT1) product-derived peptide vaccine and interferon (IFN)-β. Injected IFN-β increases major histocompatibility complex (MHC) class I molecules on tumor cells, and activates T cells and natural killer (NK) cells. Activated NK cells produced IFN-γ, which in turn activates dendritic cells (DCs), T cells, and NK cells. Under this condition, injected WT1 peptide vaccine induces WT1-specific cytotoxic T lymphocytes (CTLs) effectively. Induced WT1-specific CTLs efficiently recognize and kill MHC high-expressing tumor cells. On the other hand, IFN-β-activated NK cells kill the remaining MHC non- or low-expressing tumor cells
14.3.2 IFN-β Promotes Efficacy of WT1 Peptide Vaccination
14.3.2.1 Effect of WT1 Peptide Vaccination in Co-administration with IFN-β on Rejection of Implanted Tumors
To investigate whether IFN-β promotes tumor rejection by WT1 peptide vaccination, mice were twice treated with incomplete Freund’s adjuvant (IFA, Montanide™ ISA51)-emulsified WT1 peptide with or without IFN-β administration before implantation of WT1-expressing tumor cells (mWT1-C1498) and then WT1 peptide vaccination was repeated four times. The tumor implantation and vaccination schedules are shown in Fig. 14.6. Mice were pre-treated with i.d. injection of 100 μg WT1 peptide emulsified with IFA on days -14 and -7. On day 0, mice were subcutaneously (s.c.) implanted with 3 × 105 mWT1-C1498 cells in 100 μl of PBS at the abdomen, followed by i.d. injection of 100 μg WT1 peptide emulsified with IFA on days 1, 8, 15, and 22. During the treatment, 50,000 units of murine IFN-β was i.p. injected three times per week. Mice in control groups were treated with WT1 peptide/IFA and PBS (WT1 peptide alone group); PBS/IFA and IFN-β (IFN-β alone group); and PBS/IFA and PBS (non-treated group). Tumor growth was monitored by measuring the longest diameter of the palpable mass.
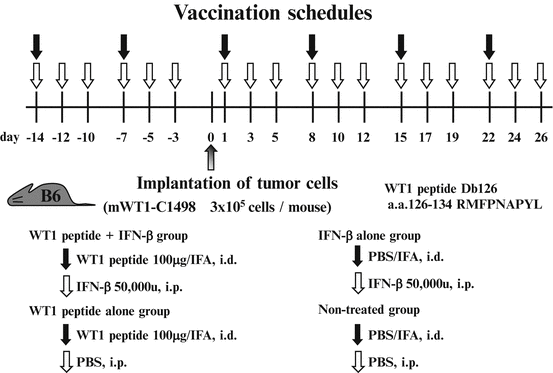

Fig. 14.6
In vivo tumor challenges and vaccination schedule. Mice were pre-treated with intradermal (i.d.) injection of 100 μg Wilms’ tumor gene (WT1) product-derived peptide emulsified with incomplete Freund’s adjuvant (IFA) on days -14 and -7. On day 0, mice were subcutaneously (s.c.) implanted with 3 × 105 mWT1-C1498 cells in 100 μl of phosphate buffered saline (PBS) in the abdomen, followed by i.d. injection of 100 μg WT1 peptide emulsified with IFA on days 1, 8, 15, and 22. During the treatment, 50,000 units of murine interferon (IFN)-β was intraperitoneally (i.p.) injected three times per week until day 26. Mice in the control groups were injected with WT1 peptide emulsified in IFA and PBS (WT1 peptide vaccine alone group), PBS emulsified in IFA and IFN-β (IFN-β alone group), or PBS emulsified in IFA and PBS (non-treated group)
Six of the 15 mice treated with WT1 peptide vaccine and IFN-β rejected implanted tumors and survived on day 75 (Fig. 14.7a). In contrast, 14 of the 15 mice treated with WT1 peptide vaccine alone, 14 of the 15 mice treated with IFN-β alone, and all of the 15 non-treated mice had died of tumor growth by day 75. Overall survival rates on day 75 were 40 % for mice treated with WT1 peptide vaccine and IFN-β, but 7, 7, and 0 % for mice treated with WT1 peptide alone, IFN-β alone, or for non-treated mice, respectively. The overall survival rates of mice treated with WT1 peptide vaccine and IFN-β were significantly higher than those of the other three groups (WT1 peptide vaccine and IFN-β vs. WT1 peptide vaccine alone, IFN-β alone, or non-treated: p < 0.05, p < 0.05, and p < 0.0005, respectively). The overall survival rates of mice treated with WT1 peptide vaccine alone or IFN-β alone were significantly higher than those of non-treated (WT1 peptide vaccine alone vs. non-treated, IFN-β alone vs. non-treated: p < 0.05 and p < 0.005, respectively). There was no significant difference in survival rate between WT1 peptide vaccine alone and IFN-β alone (Fig. 14.7b).
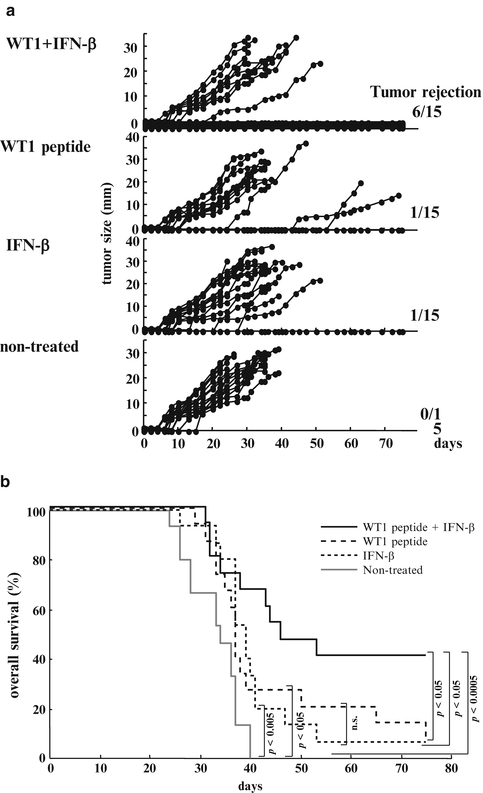

Fig. 14.7
Effect of Wilms’ tumor gene (WT1) product-derived peptide vaccination in co-administration with interferon (IFN)-β on rejection of implanted WT1-expressing tumor cells. (a) The tumor growth curves of the four groups. Each line represents tumor size of individual mice. Tumor sizes represent the longest diameters. (b) Overall survival curves of the four groups. Solid black, broken, dotted, and solid gray lines represent overall survival curves of mice treated with WT1 peptide vaccine and IFN-β, WT1 peptide vaccine alone, IFN-β alone, and non-treated mice, respectively
14.3.2.2 WT1 Peptide Vaccine and IFN-β Enhances Induction of WT1-Specific CTLs and Activation of Natural Killer (NK) Cells
In order to analyze immune responses, we performed in vivo experiments independently from those for the assessment of survival. Tumor-bearing mice treated with WT1 peptide vaccine and IFN-β, as shown in Fig. 14.6, were sacrificed on day 30 (8 days after the last vaccination). The splenocytes of each mouse were stimulated in vitro with WT1 peptide and assayed for WT1 peptide-specific CTL activity against WT1 peptide-pulsed and -unpulsed RMAS cells and for NK activity against YAC-1 cells. Splenocytes from mice treated with WT1 peptide vaccine and IFN-β showed the strongest WT1 peptide-specific cytotoxic activity compared with three control groups. It appeared that the WT1-specific CTL activities in splenocytes from IFN-β-treated or non-treated mice were endogenously induced as a result of immunologic stimulation by WT1-expressing tumor cells implanted (Fig. 14.8a). Furthermore, NK cell activity was higher in both the WT1 peptide vaccine and IFN-β, and IFN-β alone groups. These results suggested that NK activity was endogenously induced in WT1-expressing tumor-bearing mice and that this activity was enhanced by administration of IFN-β, which is a potent enhancer of NK activity (Fig. 14.8b). Taken together, these results indicated that the strongest rejection of implanted tumor cells in the mice treated with WT1 peptide vaccine and IFN-β resulted from the generation of the highest levels of both WT1-specific CTLs and NK cells.
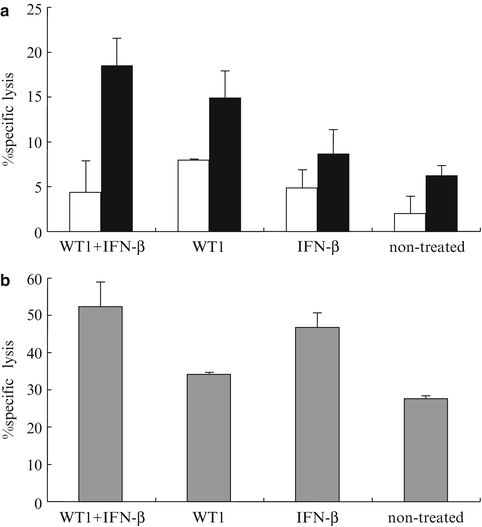

Fig. 14.8
Induction of Wilms’ tumor gene (WT1) product-derived peptide-specific cytotoxic T lymphocytes (CTLs) and enhancement of natural killer (NK) activity by co-administration of WT1 peptide vaccine and interferon (IFN)-β. Eight days after the last vaccination, splenocytes from the mice in each group were stimulated in vitro with WT1 peptide. WT1-specific CTL and NK cell activities were assayed in triplicate as cytotoxic activities against WT1 peptide-pulsed, -unpulsed RMAS, or YAC-1 cells, respectively, at the effector cell to target cell (E/T) ratio = 15. (a) WT1-specific CTL activity. Closed and open column represent cytotoxic activities against WT1 peptide-pulsed or -unpulsed RMAS, respectively. (b) NK activity is shown as cytotoxic activities against YAC-1 cells. Bars indicate standard errors
14.3.2.3 WT1-Specific CTLs and NK Cells Play Crucial Roles in the Treatment by WT1 Peptide Vaccine and IFN-β
To confirm that WT1-specific CTLs and NK cells played crucial roles in the tumor rejection, in vivo depletion of CD8+ T and/or NK cells was performed. Mice that were implanted with mWT1-C1498 cells and vaccinated with WT1 peptide and IFN-β as shown in Fig. 14.6 were treated with both or either of anti-CD8 and anti-NK monoclonal antibodies (mAbs).
Three of five mAb-non-treated mice rejected implanted tumors and survived. In contrast, all of the mice that were treated with both or either of anti-CD8 and anti-NK mAbs and vaccination-non-treated mice died of tumor development. The efficacy of WT1 peptide vaccine and IFN-β was completely cancelled by the administration of anti-CD8 and/or anti-NK mAbs. It should be noted that appearance of tumors in mice treated with both or either anti-CD8 and anti-NK mAbs was earlier than that in mAb-non-treated mice (Fig. 14.9).
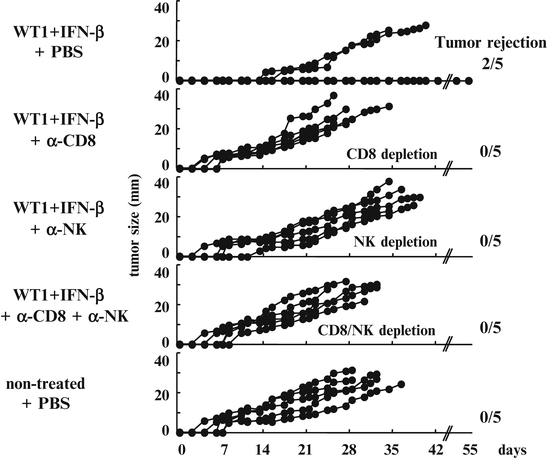

Fig. 14.9
Cancellation of the efficacy of Wilms’ tumor gene (WT1) product-derived peptide vaccine and interferon (IFN)-β by the administration of anti-CD8 and/or anti-natural killer (NK) monoclonal antibodies (mAbs). Mice were implanted with 3 × 105 mWT1-C1498 cells and treated with WT1 peptide vaccine and IFN-β as shown Fig. 14.6. The WT1- and IFN-β-treated mice were injected with phosphate buffered saline (PBS) or 200 μg of anti-CD8 and/or 200 μg of anti-NK mAbs on days -15, -8, -1, 4, 7, 11, 14, 18, 21, and 25. Each line on the tumor growth curves of the five groups represents tumor size of individual mice. Tumor sizes represent the longest diameters
These results strongly indicated that both WT1-specific CD8+ CTLs and NK cells played crucial roles in the rejection of tumor cells.
14.3.2.4 Enhancement of Major Histocompatibility Complex Class I (H-2Db) Expression on Implanted Tumor Cells by the Administration of IFN-β
Since WT1 (Db126) peptide is produced from WT1 protein through processing in tumor cells and presents on the cell surface in association with MHC class I (H-2Db) (Oka et al. 2000b), H-2Db expression levels of target cells are thought to exert a major influence on the susceptibility of the cells to attack by vaccination-induced WT1 (Db126)-specific CTLs. For this reason, the H-2Db expression levels on the implanted tumor cells (WT1-expressing C1498 cells) were examined. Tumor-bearing mice were sacrificed 30 days after tumor cell implantation, the tumors were resected, and the tumor cells were stained with anti-H-2Db antibody (Fig. 14.10). The expression levels of H-2Db on tumor cells was significantly higher in mice treated with WT1 peptide vaccine and IFN-β or IFN-β alone than in those treated with WT1 peptide vaccine alone or non-treated mice (p < 0.05) (Fig. 14.10b). These results indicated that IFN-β administration enhanced the expression of H-2Db on tumor cells, which should make tumor cells more susceptible to attack by WT1-specific CTLs.
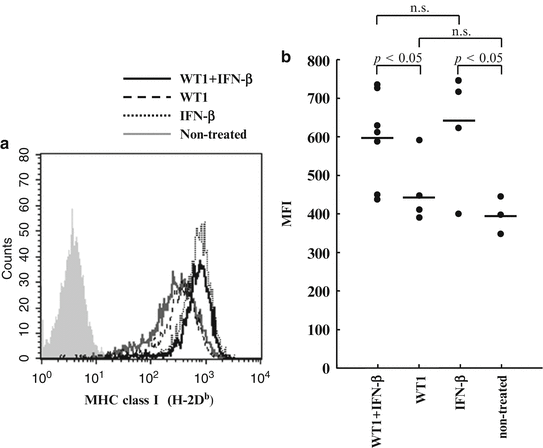

Fig. 14.10
Interferon (IFN)-β enhanced major histocompatibility complex (MHC) class I (H-2Db) expression of tumor cells in vivo. (a) H-2Db expression levels of tumor cells recovered from mice. Solid black, broken, dotted, and solid gray lines represent the expression levels of tumor cells from mice treated with Wilms’ tumor gene (WT1) product-derived peptide vaccine and IFN-β, WT1 peptide vaccine alone, or IFN-β alone, and non-treated mice, respectively. (b) The mean fluorescence intensity (MFI) of H-2Db expression of tumor cells from mice
14.4 Conclusion and Future Works
Co-administration of appropriate immunopotentiating agents, including adjuvants or cytokines, together with WT1 peptide vaccine could enhance its therapeutic efficacy. WT1 peptide vaccine combined with BCG-CWS enhanced both innate and WT1-specific immune responses (acquired immunity). Co-administration of WT1 peptide vaccine and IFN-β enhanced the induction of WT1-specific CTLs, NK activity, and MHC class I expression on the tumor cells. These synergistic effects increased the survival rate of WT1 peptide vaccine and IFN-β treated mice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree