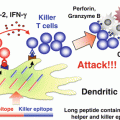
Fig. 20.1
Mechanisms for the differential immune status among cancer patients. Balance of the anti-tumor T cell induction pathway and immunosuppressive pathway along with environmental factors may define the differences in T cell immune status among cancer patients. Passenger mutations may induce anti-tumor T cells, while driver mutations promote immunosuppressive cascades
In the positive anti-tumor immune pathway, various factors are involved in its strength. We have previously shown that TILs recognize mutated peptides derived from genome DNA mutations (Robbins et al. 1996; Kawakami et al. 2001) of cancer cells. It was recently shown that melanoma TILs frequently recognize such mutated peptides derived from passenger mutations by systemic exome DNA sequencing, and that these immunogenic mutations are different among patients (Robbins et al. 2013), indicating a possible necessity for personalized therapies. We have reported that immune responses occur to the frameshift mutations caused by DNA slippage through dysfunction of DNA mismatch repair enzymes such as MLH1 (mutL homolog 1) in MSI (microsatellite instability)+ colon cancer (Ishikawa et al. 2003). Interestingly, the patients with MSI+ colon cancer have high CD8+ T cell infiltration in tumors, and relatively good prognosis after surgery even though they show a histologically malignant appearance. Therefore, differences in DNA mutation status in cancer cells may result in the different T cell responses. It was also shown that loss of production of a cytokine (IL-15) and chemokine [chemokine (C-X-C motif) ligand (CXCL) 13] through DNA deletions in cancer cells cause fewer T cell infiltrations in colon cancer (Bindea et al. 2013; Mlecnik et al. 2014). In addition to these cancer cell characteristics, difference in patients’ immune reactivity partly defined by polymorphism of immune systems including HLA type, may also influence the anti-tumor T cell induction pathway.
In the negative immunosuppressive pathway, we have previously shown that simple overproduction of immunosuppressive molecules such as transforming growth factor (TGF)-β in tumor affects the immune status of tumor microenvironments (e.g., DC impairment, increase of Tregs and MDSCs in tumors and draining lymph nodes) and subsequent decrease of anti-tumor T cell induction and accumulation in tumors (Nakamura et al. 2014). In addition, we have shown that oncogene activation (e.g., driver mutations, over-expression) rather promotes multiple immunosuppressive cascades and generates immunosuppressive tumor microenvironments. Oncogene activation status is different in cancer patients, and the immunosuppressive pathway is also possibly influenced by polymorphisms in the immunosuppressive pathway. These factors may define the strength of the immunosuppressive pathway for anti-tumor T cell responses. Altogether, cancer cell characteristics (e.g., gene alterations), patients’ immune reactivity (e.g., immune polymorphism), and environmental factors (e.g., microbiota, diet, smoking) may generate the differential T cell immune status among cancer patients.
20.5 Immune Biomarkers for Cancer Therapies
There are differences in immune status, including T cells, B cells, and various myeloid cells, in cancer patients, and it may be correlated with patients’ prognosis after cancer therapies, indicating that these are immune biomarkers for various cancer therapies, including immunotherapies.
Recently PD-1/PD-L1 blockade therapy [e.g., anti-PD-1 antibody (Ab) anti-PD-L1 Ab] showed durable clinical responses in patients with advanced cancers, including melanoma, kidney cancer, lung cancer, bladder cancer, ovarian cancer, head and neck cancer, and Hodgkin disease. In the search for immune biomarkers for the PD-1/PD-L1 blockade therapy, PD-L1 expression on cancer cells as well as CD8+ T cell infiltrations in tumors were found to correlate with good response to anti-PD-1 Ab treatment (Topalian et al. 2013). Interestingly, PD-L1 on cancer cells appears to be induced by interferon (IFN)-γ produced by tumor-infiltrating CD8+ T cells in melanoma (Taube et al. 2014). Combination of both PD-L1 expression and CD8+ T cell tumor infiltration appears to be a better immune biomarker to predict response to the PD-1 Ab therapy in melanoma. T cells induced against mutated peptides may reject melanoma cells after PD-1/PD-L1 blockade. Numbers of DNA mutations are much higher in UV-related melanoma and smoking-related lung cancer, which are relatively responsive cancers to the PD-1/PD-L1 blockade, suggesting again that mutated peptide-specific T cells may be involved in the PD-1/PD-L1 therapies. In this regard, MSI+ colon cancer, which also has frequent DNA mutations, may be a good candidate for the PD-1/PD-L1 therapies. These observations indicate that pre-existing anti-tumor T cell immunity may be essential for the PD-1/PD-L1 blockade therapy. CD8+ T cell infiltrations were also reported to correlate with responses to cancer vaccines and IL-2 therapy in melanoma.
T cell infiltration in tumors is also a possible immune biomarker for other cancer therapies in various cancers. It is correlated with favorable prognosis of colon cancer patients after curative surgery and is possibly a better prognostic marker than TNM (tumor, nodes, metastasis) staging criteria (Pagès et al. 2009). There is also a report showing that T cell infiltrations in liver metastases of colon cancer is correlated with chemotherapy response. Similarly, we and others have shown that T cell infiltration correlates with favorable prognosis after various cancer therapies including surgery, radiation, and chemotherapy in various cancers (e.g., lung, ovarian, and cervical cancer). In addition to TILs, plasma cytokines such as IL-6 and IL-8 are reported to be associated with responses to various cancer therapies, including chemotherapy and molecular target therapy. Therefore, immune status is a possible biomarker for various cancer therapies. Further confirmation in a large numbers of patients and consideration of inclusion of these immune biomarkers into current prognostic diagnosis such as TNM staging criteria are warranted (Fridman et al. 2012).
20.6 Combination Immunotherapy
The immune-checkpoint blockade and T cell-based ACT are not effective in all patients. It is important to increase the anti-tumor effects of these immunotherapies. In PD-1/PD-L1 therapy, both PD-L1 expression on cancer cells and CD8+ T cell infiltration in tumors are correlated with good response. Two strategies should be considered: one is to increase the efficacy in the patients with both T cell infiltration and PD-L1 expression, the other is to change the immune status of the non-responders (without T cell infiltration and PD-L1 expression) to responsive immune status (e.g., induction of anti-tumor T cells and accumulation into tumors). We have previously identified key regulation points in the anti-tumor T cell responses through the identification of human tumor antigens recognized by TILs (Kawakami et al. 1994a, b, c), and analyses of anti-tumor antigen-specific T cell responses in patients in immunotherapy clinical trials (Salgaller et al. 1995; Rosenberg et al. 1998). We proposed the development of following methods for modulation of the different key regulation points, and their appropriate combinations for effective cancer immunotherapy (Kawakami et al. 2013b) (Fig. 20.2): (1) identification of better tumor antigens such as the antigens involved in cancer cell proliferation and survival, and expressed in cancer-initiating cells [e.g., Wilm’s tumor gene 1 (WT-1), SOX2, SOX6); (2) in situ tumor destruction methods to induce immune response to endogenous tumor antigens including unique mutated peptides (immunogenic cancer cell death) (e.g., chemotherapy, molecular targeted drugs, anti-tumor Ab, irradiation, cryoablation, radiofrequency ablation, oncolytic viruses); (3) enhancing methods for antigen-presenting function of DCs [e.g., adjuvants (Toll-like receptor (TLR)/stimulator of IFN genes (STING)], cytokines [IL-12, tumor necrosis factor (TNF)-α], agonistic antibodies (anti-CD40 Ab); (4) in vivo activation and expansion methods for anti-tumor T cells [e.g., cytokines (IL-2, IL-7, IL-15, IL-21), agonistic antibodies against co-stimulatory molecules on T cells (anti-CD134, CD137 Ab), T cell-based ACT]; (5) reversal methods for cancer-induced immunosuppression [neutralizing and depleting antibodies (e.g., TGF-β, Τreg), immune-checkpoint blockers (e.g., anti-CTLA-4, anti-PD-1/PD-L1, anti-LAG3, anti-TIM3), chemotherapy, molecular-targeted drugs (e.g., tyrosine kinase inhibitors, transcription factor inhibitors)].
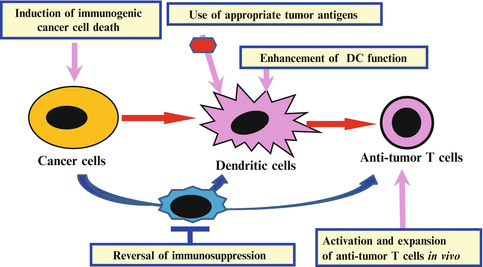

Fig. 20.2
Combinations of immune interventions targeting key regulation points in the anti-tumor T cell responses. Development of immune interventions targeting different key regulation points in the anti-tumor immune responses and their appropriate combination is important to develop effective cancer immunotherapy
We have been working on these immune-modulating methods, including the identification of better tumor antigens (e.g., SOX6, mutant BRAF) (Ueda et al. 2004, 2010), DC-stimulating new adjuvants [e.g., Mycobacterium bovis Bacillus Calmette-Guérin–cell wall skeleton (BCG-CWS)] (Udagawa et al. 2006), oncolytic herpes simplex virus (HSV) (Toda et al. 2002; Ohkusu-Tsukada et al. 2011), new TLR-stimulating compounds, cultured anti-tumor T cells (e.g., anti-tumor TILs, new TCR/CAR transduced T cells), various molecular-targeted drugs which act on both cancer cells, and activating and inhibiting immune cells (Iwata-Kajihara et al. 2011; Yaguchi et al. 2012; Kudo-Saito et al. 2012; Nishio et al. 2014; Sumimoto et al. 2006). We have previously reported that mutant BRAF depletion not only inhibits cell proliferation and invasion of human melanoma cells, but also inhibits production of multiple immunosuppressive cytokines such as IL-10, IL-6, and vascular endothelial growth factor (VEGF). Inhibitory effects of human melanoma cells on DC functions such as IL-12 production and T cell-stimulatory ability was reduced by mutant BRAF depletion in melanoma cells by specific short hairpin RNAs (shRNAs) or mitogen-activated protein kinase (MAPK) signaling inhibitors such as MAPK kinase (MEK) inhibitors. Interestingly, it has recently been reported that administration of selective mutant BRAF inhibitors alone induces abundant CD8+ T cell accumulation in regressing tumors, but not in progressing tumors (Wilmott et al. 2012). Administration of BRAF inhibitors appears to augment anti-tumor CD8+ T cells through multiple mechanisms, including immunogenic cancer cell death to release immunogenic antigens, enhanced expression of melanoma antigens [melanocyte differentiation antigens MART-1 (melanoma antigen recognized by T cells 1), gp100], and inhibition of production of multiple immunosuppressive cytokines, while the mutant BRAF selective inhibitor had fewer T cell inhibitory effects. We have shown that STAT3 (signal transducer and activator of transcription 3) has similar properties. In addition, immunosuppressive cytokines produced by melanoma cells impair immune cells by activating STAT3 and make them immunosuppressive cells such as tolerogenic DCs and immunosuppressive macrophages. Therefore, administration of STAT3 inhibitors may also be useful for augmentation of anti-tumor T cell immunity (Iwata-Kajihara et al. 2011). We have shown that some combinations increase the efficacy of immunotherapies in mouse tumor models, including cryoablation plus intratumoral injection of TLR stimulating BCG-CWS pretreated cultured DCs (Udagawa et al. 2006) and molecular-targeted drugs plus PD-1/PD-L1 blockade.
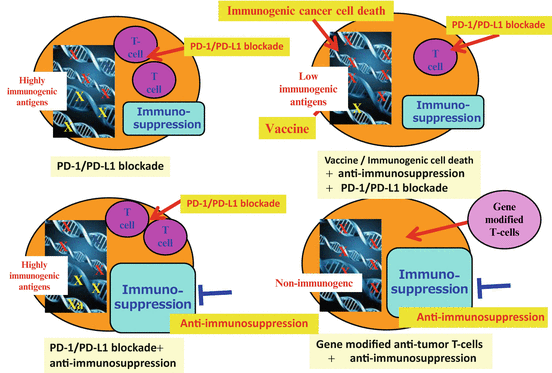
20.7 Personalized Immunotherapies Based on the Patients’ Immune Status
Immune status varies among cancer patients, and it correlates with responses to cancer therapies, including immunotherapies, indicating that personalized therapy should be exploited for cancer immunotherapy based on the analysis of individual patients’ immune status (Fig. 20.3). For example, in patients with pre-existing anti-tumor T cell immunity and PD-1-/PD-L1-related immune suppression, PD-1/PD-L1 blockade is a reasonable strategy. If immune suppression mechanisms other than PD-1/PD-L1 are dominant, other methods to overcome the immunosuppression are needed even for patients with T cell immunity. For colon cancer patients, the PD-1/PD-L1 therapies have not shown good responses. This may be explained by either the fact that TILs are not tumor-specific T cells or that immunosuppression mechanisms other than PD-1/PD-L1 are dominant. For the patients with less T cell response but who still have immunogenic tumor antigens, immune-stimulating interventions [e.g., immunogenic cancer cell death to release immunogenic antigens, cancer vaccine with strong adjuvants, T cell-stimulating reagents (e.g., agonistic Ab, anti-CTLA Ab)] may promote T cell immunity and make it more responsive to the PD-1/PD-L1 blockade. For patients with almost no immunogenic antigens, ACT with artificially generated anti-tumor T cells such as TCR /CAR-transduced T cells may be applied. Altogether, personalized combination immunotherapy should be considered for future improvement in cancer immunotherapy.