Fig. 6.1
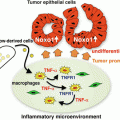
Anchorage-dependent multicellular aggregate (MCA) formation. When adult T cell leukemia/lymphoma (ATL) cells are directly co-cultured with epithelial-like feeder cells overnight, the majority of ATL cells adhere to the feeder layer and form MCAs in an anchorage-dependent manner. ATL cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) prior to coculture
6.4.2 Emergence of Quiescent CD44 High CSC-Like ATL Cells Through Anchorage-Dependent MCA Formation
Co-culture with epithelial-like feeder cells greatly increased the proportion of ATL cells in the resting G0/G1 phase and decreased the proportion of Ki-67+ proliferating cells, suggesting that the majority of MCA-forming ATL cells entered a quiescent state. At the same time, ATL cells forming MCAs began to express CD44 strongly. In particular, ATL cells located at the top of MCAs were strongly positive for intracellular CD44 (Fig. 6.2). When co-cultured with feeder cells, primary leukemia cells isolated from ATL patients showed similar MCA formation and CD44 staining patterns regardless of whether the patients were suffering from chronic-type or acute-type ATL. By contrast, when ATL cells were co-cultured with mesenchymal feeder cells, they neither formed MCAs nor increased CD44 expression. Similar results were obtained when HUT78 cells, an epidermotropic HTLV-1-negative T cell lymphoma cell line established from a patient with Sezary syndrome, were co-cultured with HEK293T cells. Interestingly, when certain pancreatic cancer cells were co-cultured with HEK293T cells, they also formed MCAs and increased CD44 expression (unpublished data). Therefore, co-culture with epithelial-like cells may induce anchorage-dependent MCA formation and the emergence of CD44 high CSC-like cells in a broad range of tumor cells.
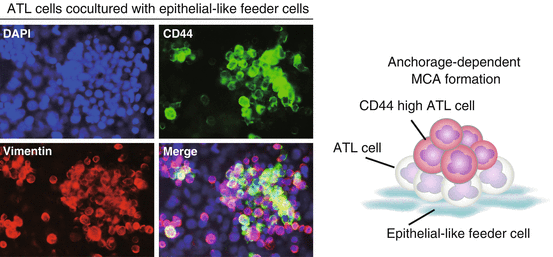

Fig. 6.2
Multicellular aggregate (MCA)-forming adult T cell leukemia/lymphoma (ATL) cells are quiescent and express high levels of CD44. Anchorage-dependent MCA formation induces CD44 high ATL cells. CD44 high cells are located primarily at the upper and central regions of aggregates
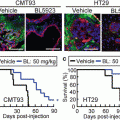
6.4.3 Aggregate-Forming ATL Cells Acquire Resistance to Histone Deacetylase Inhibitor-Induced Apoptosis
When ATL cells were cultured alone in the presence of histone deacetylase (HDAC) inhibitors such as trichostatin A (TSA), valproic acid sodium salt and sodium butyrate, they showed a dose-dependent increase in the percentage of apoptotic cells. By contrast, ATL cells co-cultured with HEK293T cells escaped from HDAC inhibitor-induced apoptosis, suggesting that anchorage-dependent MCA formation induces resistance to HDAC inhibitor-induced apoptosis in ATL cells (Fig. 6.3). HDAC inhibitors have emerged as a new class of promising chemotherapeutic agents against cancer (Marks et al. 2000; West and Johnstone. 2014). Vorinostat, also known as suberanilohydroxamic acid, has been approved by the US Food and Drug Administration for the treatment of cutaneous T cell lymphoma (Mann et al. 2007). However, monotherapeutic and combined-therapeutic clinical trials with HDAC inhibitors have had only limited success in most types of cancers (Olsen et al. 2007; Piekarz et al. 2011). Our study suggests that the therapeutic efficacy of HDAC inhibitors will be reduced against leukemia/lymphoma cells that have invaded into epithelial tissues.
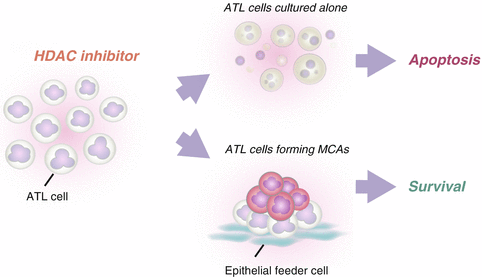

Fig. 6.3
Multicellular aggregate (MCA)-forming adult T cell leukemia/lymphoma (ATL) cells are resistant to histone deacetylase (HDAC) inhibitor-induced apoptosis. When ATL cells are exposed to HDAC inhibitors, they undergo apoptosis in a dose-dependent manner. However, when they are exposed to HDAC inhibitors in the presence of epithelial-like feeder cells, they do not undergo apoptosis
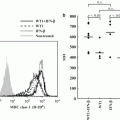
6.4.4 Anchorage-Dependent MCA Formation Suppresses Genomic Instabilities in ATL Cells
HTLV-1 proteins are generally undetectable in HTLV-1-infected cells isolated from HTLV-1 carriers because of viral gene silencing. Such silencing is observed not only in asymptomatic carriers but also in ATL patients (Furukawa et al. 1995; Taniguchi et al. 2005). Recent work has shown that type I interferon-induced HTLV-1 gag expression is suppressed in an ATL cell line when it is co-cultured with stromal cells (Kinpara et al. 2009). Epigenetic regulations are also involved in viral gene silencing in ATL cells (Ego et al. 2002; Michael et al. 2006; Mosley et al. 2006). Treatment with HDAC inhibitors can activate viral genes in ATL cells in single cell culture in vitro (Ego et al. 2002; Mosley et al. 2006). However, when ATL cells were co-cultured with HEK293T cells, treatment with TSA failed to increase the transcriptional activity of the HTLV-1 long terminal repeat, indicating that co-culture with epithelial-like feeder cells can block TSA-induced viral gene reactivation. Taken together, viral gene expression in ATL cells may be suppressed by the dual action of the microenvironment of the host–tumor interface and epigenetic regulation in host cells. HDAC inhibitors induce transcriptional activation of viral and host genes as well as genomic instability by a variety of mechanisms. Thus, our data suggest that epithelial-like feeder cells play a key role in protecting ATL cells from genomic instability, including the reactivation of viral genes.
6.5 Concluding Remarks
Metastatic cancer cells occur as both single cells and MCAs in several solid tumors (Hudson et al. 2008), pointing to the importance of MCA formation. However, the mechanism underlying MCA formation is poorly understood. Recent work indicates that MCA formation determines gene expression, proliferation, drug resistance, and immune escape in vitro in follicular lymphoma (Gravelle et al. 2014), suggesting that it affects the malignant potential of the tumor. Anchorage-dependent MCA formation discussed in this chapter involves both cell–cell interactions between ATL cells and cell–cell interactions between MCA-forming ATL cells and epithelial-like feeder cells. Both types of cell–cell interactions are presumably important for ATL cells to acquire intractable CSC-like phenotypes.
In summary, we suggest that the intractability of tissue-infiltrating ATL cells may be partly accounted for by the acquisition of CSC-like phenotypes. The simple co-culture system we have described here should be useful for dissecting the tumor microenvironment in vitro. It may also provide insights into the nature of CSCs and the CSC niche.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Competing Financial Interests
The authors declare no competing financial interests.
References
Ben-Porath I, Thomson MW, Carey VJ et al (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40(5):499–507CrossRefPubMedCentralPubMed
Bourguignon LY, Peyrollier K, Xia W et al (2008) Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 283(25):17635–17651CrossRefPubMedCentralPubMed
Brown RL, Reinke LM, Damerow MS et al (2011) CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest 121(3):1064–1074CrossRefPubMedCentralPubMed
Catalina P, Montes R, Ligero G et al (2008) Human ESCs predisposition to karyotypic instability: is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer 7:76CrossRefPubMedCentralPubMed
Chagan-Yasutan H, Tsukasaki K, Takahashi Y et al (2011) Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk Res 35(11):1484–1490CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree