Fig. 9.1
Cross-talk between hepatitis C virus (HCV)-infected hepatocytes and hepatic stellate cells (HSCs) induces macrophage inflammatory protein (MIP)-1β expression. (a) Hepatocytes or HCV-infected hepatocytes were cultured alone or in the presence of HSCs for 24 h. The expression of MIP-1β was measured by quantitative real-time polymerase chain reaction (qRT-PCR). (b) Hepatocytes or HCV-infected hepatocytes were treated with conditioned medium from hepatocytes (Hepatocyte-CM) or HSCs (HSC-CM) for 24 h. The expression of MIP-1β was measured by qRT-PCR. (c) HSCs were treated with transforming growth factor (TGF)-β1 for 24 h. Hepatocytes or HCV-infected hepatocytes were treated with HSC-CM or TGF-β1-stimulated HCS-CM for 24 h. The expression of MIP-1β was measured by qRT-PCR
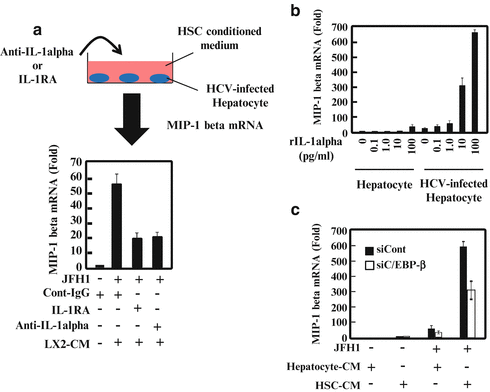
Fig. 9.2
Interleukin (IL)-1α and CCAAT (cytosine–cytosine–adenosine–adenosine–thymidine)-enhancer-binding protein (C/EBP)-β contribute to the hepatitis C virus (HCV)-infected hepatocyte response to hepatic stellate cells (HSCs). (a) HCV-infected hepatocytes were cultured with HSCs together with an isotype control, anti-IL-1, or IL-1 receptor antagonist (IL-1RA) for 24 h. Macrophage inflammatory protein (MIP)-1 expression was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). (b) Hepatocytes or HCV-infected hepatocytes were treated with various amounts of recombinant IL-1 (0, 0.1, 1.0, 10, or 100 pg/ml) for 24 h. MIP-1 expression was analyzed by qRT-PCR. (c) Hepatocytes and HCV-infected hepatocytes were transfected with control small-interfering RNA (siRNA) or small-interfering C/EBP (siC/EBP)-β. After 24 h of transfection, the cells were treated with conditioned medium from hepatocytes (Hepatocyte-CM) or HSCs (HSC-CM) for 24 h. MIP-1 expression was analyzed by qRT-PCR
These results suggest that TGF-β, which is induced by HCV infection, stimulates quiescent HSCs, and activation or trans-differentiation of HSCs leads to the expression of IL-1α, resulting in increased levels of inflammatory cytokines and chemokines in HCV-infected hepatocytes (Fig. 9.3).
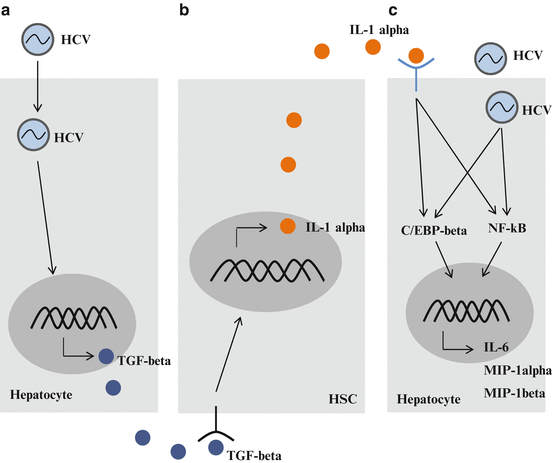

Fig. 9.3
Cross-talk between hepatitis C virus (HCV)-infected hepatocytes and hepatic stellate cells (HSCs). (a) HCV infection induces the expression of growth factors, such as transforming growth factor (TGF)-β. (b) When quiescent HSCs are activated by growth factors, activated HSCs secrete inflammatory cytokines, such as interleukin (IL)-1α. (c) HCV-infected cells are stimulated by IL-1α. An increase in inflammatory cytokine production accelerates inflammation in the liver
References
Brun P et al (2005) Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 289:571–578CrossRef
Burdette D et al (2012) Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J Gen Virol 93:235–246CrossRefPubMedCentralPubMed
Chang ML et al (2007a) Topological and evolutional relationships between HCV core protein and hepatic lipid vesicles: studies in vitro and in conditionally transgenic mice. World J Gastroenterol 13:3472–3477CrossRefPubMedCentralPubMed
Chang ML et al (2009) Hepatic inflammation mediated by hepatitis C virus core protein is ameliorated by blocking complement activation. BMC Med Genomics 2:51CrossRefPubMedCentralPubMed
Chen W et al (2014) HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS One 9:e84953CrossRefPubMedCentralPubMed
Coulouarn C et al (2012) Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res 72:2533–2542CrossRefPubMedCentralPubMed
Duffield JS et al (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115:56–65CrossRefPubMedCentralPubMed
Friedman SL (2008a) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88:125–172CrossRefPubMedCentralPubMed
Friedman SL (2008b) Mechanisms of hepatic fibrogenesis. Gastroenterology 2008(134):1655–1669CrossRef
Jing Y et al (2011) Tumor necrosis factor-alpha promotes tumor growth by inducing vascular endothelial growth factor. Cancer Invest 29:485–493PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree