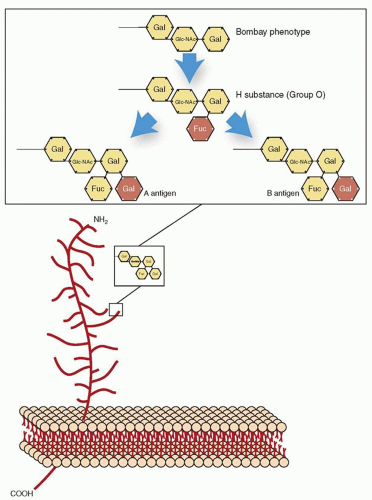
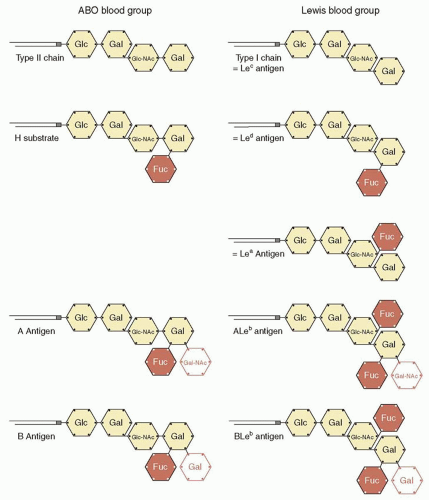
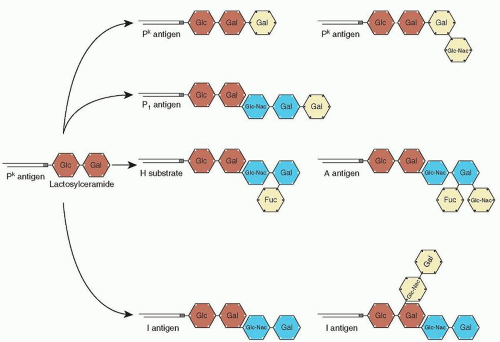
|
|
|
|
Gene Name(s) |
ISBT No. |
System Name (symbol) |
No. of Antigens |
Antigen(s) |
ISBT |
HGNC |
Gene Product(s) |
Chromosomal Location |
001 |
ABO (ABO) |
4 |
A, B, AB, A1 |
ABO |
ABO |
A = α-3-N-acetyl-D-galactosaminyltransferase |
9q34.2 |
|
|
|
|
|
|
B = α-3-D-galactosyltransferase |
002 |
MNS (MNS) |
46 |
M, N, S, s, U, He, Mia, Mc, Vw, Mur, Mg, Vr, Me, Mta, Sta, Ria, Cla, Nya, Hut, Hil, Mv, Far, sD, Mit, Dantu, Hop, Nob, Ena, EnaKT, N′, Or, DANE, TSEN, MINY, MUT, SAT, ERIK, Osa, ENEP, ENEH, HAG, ENAV, MARS, ENDA, ENEV, MNTD |
MNS |
GYPA
GYPB
GYPE |
Glycophorin A (GYPA)
Glycophorin B (GYPB)
Glycophorin E (GYPE) |
4q31.21 |
003 |
P (P1) |
3 |
|
P1, PK, NOR |
P1 |
|
22q11.2-qter |
004 |
Rh (RH) |
54 |
D, C, E, c, e, f, Ce, Cw, Cx, V, Ew, G, Hro, Hr, hrs, VS, CG, CE, Dw, c-like, cE, hrH, Rh29, Goa, hrB, Rh32, Rh33, HrB, Rh35, Bea, Evans, Rh39, Tar, Rh41, Rh42, Crawford, Nou, Riv, Sec, Dav, JAL, STEM, FPTT, MAR, BARC, JAHK, DAK, LOCR, CENR, CEST, CELO, CEAG, PARG, CEVF |
RH |
RHD
RHCE |
Acetylated RhD protein
Acetylated RhCE protein |
1p36.11 |
005 |
Lutheran (LU) |
20 |
Lua, Lub, Lu3, Lu4, Lu5, Lu6, Lu7, Lu8, Lu9, Lu11, Lu12, Lu13, Lu14, Lu16, Lu17, Aua, Aub, Lu20, Lu21, LURC |
LU |
B-CAM |
B-cell adhesion molecule |
19q12-q13 |
006 |
Kell (KEL) |
35 |
K, k, Kpa, Kpb, Ku, Jsa, Jsb, Ula, K11, K12, K13, K14, K16, K17, K18, K19, Km, Kpc, K22, K23, K24, VLAN, TOU, RAZ, VONG, KALT, KTIM, KYO, KUCI, KANT, KASH, KELP, KETI, KHUL, KYOR |
KEL |
KEL |
Zinc endopeptidase |
7q33 |
007 |
Lewis (LE) |
6 |
Lea, Leb, Leab, LebH, ALeb, BLeb |
LE |
FUT3 |
α-1,3/1,4-L-Fucosyltransferase |
19p13.3 |
008 |
Duffy (FY) |
6 |
Fya, Fyb, Fy3, Fy4, Fy5, Fy6 |
FY |
DARC |
Duffy antigen receptor for chemokines |
1q21-q22 |
009 |
Kidd (JK) |
3 |
Jka, Jkb, JK3 |
JK |
SLC14A1 |
Urea transporter |
18q11-q12 |
010 |
Diego (DI) |
22 |
Dia, Dib, Wra, Wrb, Wda, Rba, WARR, ELO, Wu, Bpa, Moa, Hga, Vga, Swa, BOW, NFLD, Jna, KREP, Tra, Fra, SW1, DISK |
DI |
SLC4A1 |
Anion exchanger 1, solute carrier family 4/band 3 |
17q12-q21 |
011 |
Yt (YT) |
2 |
Yta, Ytb |
YT |
ACHE |
Acetylcholinesterase |
7q22 |
012 |
Xg (XG) |
2 |
Xga, CD99 |
XG |
XG |
Xga glycoprotein |
Xp22.32 |
013 |
Scianna (SC) |
7 |
Sc1, Sc2, Sc3, Rd, STAR, SCER, SCAN |
SC |
ERMAP |
Erythrocyte membrane-associated protein (ERMAP) |
1p34 |
014 |
Dombrock (DO) |
8 |
Doa, Dob, Gya, Hy, Joa, DOYA, DOMR, DOLG |
DO |
ART4 |
ADP-ribosyltransferase 4 |
12p13.2-q13.3 |
015 |
Colton (CO) |
4 |
Coa, Cob, Co3, Co4 |
CO |
AQP1 |
Aquaporin-1 (AQP1) |
7p14 |
016 |
Landsteiner-Wiener (LW) |
3 |
LWa, LWab, LWb |
LW |
ICAM4 |
Intracellular adhesion molecule 4 (ICAM4) |
19p13.2-cen |
017 |
Chido/Rodgers (CH/RG) |
9 |
Ch1, Ch2, Ch3, Ch4, Ch5, Ch6, WH, Rg1, Rg2 |
CH/RG |
C4B/C4A |
Complement component 4A protein[en]Complement component 4B protein |
6p21.3 |
018 |
H (H) |
1 |
H |
H |
FUT1 |
Galactoside 2-α-L-fucosyltransferase 1 |
19q13.1-qter |
019 |
Kx (XK) |
1 |
Kx |
KX |
XK |
Membrane transport protein XK |
Xp21.1 |
020 |
Gerbich (GE) |
11 |
Ge2, Ge3, Ge4, Wb, Lsa, Ana, Dha, GEIS, GEPL, GEAT, GET1 |
GE |
GYPC |
Glycophorin C (GPC) and GPD (glycophorin C precursor) |
2q14-q21 |
021 |
Cromer (CROM) |
18 |
Cra, Tca, Tcb, Tcc, Dra, Esa, IFC, WESa, WESb, UMC, GUTI, SERF, ZENA, CROV, CRAM, CROZ, CRUE, CRAG |
CROM |
CD55 |
CD55/decay accelerating factor (DAF) |
1q32 |
022 |
Knops (KN) |
9 |
Kna, Knb, McCa, S11, Yka, McCb, S12, S13, KCAM |
KN |
CR1 |
CD35/CR1 |
1q32 |
023 |
Indian (IN) |
4 |
Ina, Inb, INFI, INJA |
IN |
CD44 |
CD44 |
11p13 |
024 |
Ok (OK) |
3 |
Oka, OKGV, OKVM |
OK |
BSG |
Basigin |
19p13.3 |
025 |
Raph (RAPH) |
1 |
MER2 |
RAPH |
CD151 |
CD151 |
11p15.5 |
026 |
John Milton Hagen (JMH) |
6 |
JMH, JMHK, JMHL, JMHG, JMHM, JMHQ |
JMH |
SEMA7A |
Semaphorin 7A |
15q22.3-q23 |
027 |
I (I) |
1 |
I |
I |
GCNT2 |
I- β-1, 6-N-acetylglucosaminyltransferase A |
6p24.2 |
028 |
Globoside (GLOB) |
1 |
P |
GLOB |
B3GALNT1 |
UDP-N-acetyl-galactosamine globo-triaosylceramide 3-β–N acetylgalactosaminyl-transferase |
3q25 |
029 |
Gill (GIL) |
1 |
GIL |
GIL |
AQP3 |
Aquaporin-3 (AQP3) |
9p13 |
030 |
Rh-associated glycoprotein (RHAG) |
4 |
Duclos, Ola, DSLKa, RHAG 4 |
RHAG |
RHAG |
|
6p12.3 |
031 |
Forssman (FOR) |
1 |
FORS1 |
GBGT1 |
GBGT1 |
globoside alpha-1,3-N-acetylgalactosaminyltransferase 1 |
9q34.13-q34.3 |
032 |
JR |
1 |
Jra |
ABCG2 |
ABCG2 |
ATP-binding cassette, sub-family G (WHITE), member 2 |
4q22.1 |
033 |
LAN |
1 |
LAN |
ABCB6 |
ABCB6 |
ATP-binding cassette, sub-family B (MDR/TAP), member 6 |
2q36 |
HCNC, HUGO gene nomenclature committee (www.genenames.org); ISBT, International Society of Blood Transfusion; No, number. |
Data from Daniels GL, Fletcher A, Garratty G, et al. International Society of Blood Transfusion Working Party on terminology for red cell surface antigens. Vox Sang 2004;87:304-316; Denomme GA, Rios M, Reid ME. Molecular protocols in transfusion medicine. San Diego, CA: Academic Press, 2000; Logdberg L, Reid MA, Lamont RE, et al. Human Blood Group Genes 2004: chromosomal locations and cloning strategies. Transfus Med Rev 2005;19:45-57; Costa FP, Hue-Roye K, Sausais L, et al. Absence of DOMR, a new antigen in the Dombrock blood group system that weakens expression of Do(b), Gy(a), Hy, Jo(a), and DOYA antigens, Transfusion 2010; 50:2026-2031; Smart EA and Storry JR. The OK blood group system: a review. Immunohematology 2010; 26:124-126; Walker PS, Reid ME. The Gerbich blood group system: a review. Immunohematology 2010; 26:124-126; and International Society of Blood Transfusion Working Party on terminology for red cell antigens web site: http://www.isbtweb.org/fileadmin/user_upload/WP_on_Red_Cell_Immunogenetics_and/Updates/Table_of_blood_group_antigens_within_systems_v3_2_130331.pdf Accessed April 3, 2013 |
|