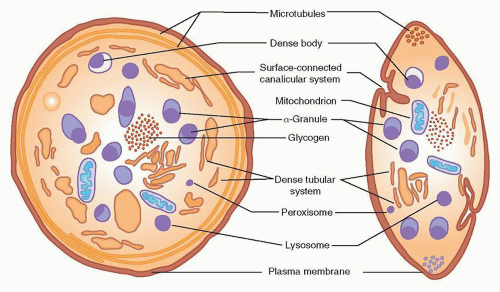
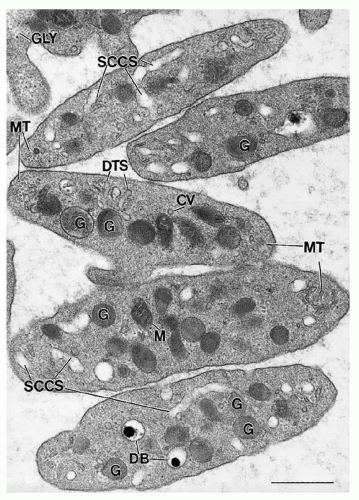
Platelets possess secretory granules and mechanisms for cargo release to amplify responses to stimuli and influence the surrounding environment. Platelet granule structures include α– and dense granules, lysosomes, and peroxisomes. α-Granules and the dense bodies are the main secretory granules that release cargo (e.g., fibrinogen and adenosine diphosphate [ADP]) upon platelet activation.
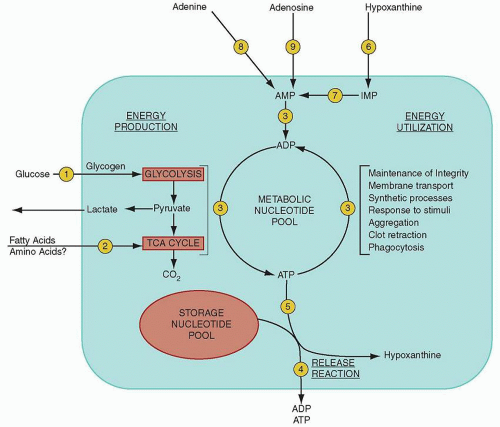
Platelet granule secretion begins with a dramatic increase in platelet metabolic activity, set off by a wave of calcium release and marked by increased adenosine triphosphate (ATP) production.
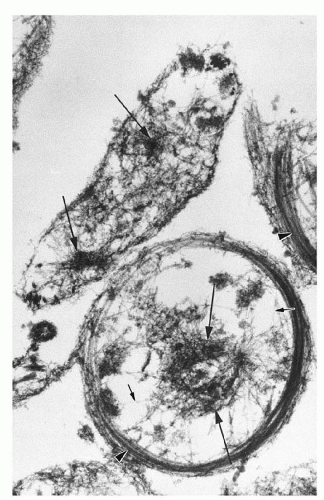
59 After platelet stimulation by agonists, a “contractile ring” develops around centralized granules,
5,6 the granules fuse with the surface membranes, and then they extrude their contents.
60 The molecular events underlying platelet granule release involve many of the same proteins and processes observed in other systems of membrane docking, fusion, and extrusion.
61,62 Granule secretion in platelets is a graded process that depends on the number, concentration, and nature of the original stimulus/stimuli, either strong (e.g., thrombin and collagen) or weak (e.g., ADP and epinephrine).
62
α-Granules
α-Granules, with a cross-sectional diameter of approximately 300 nm and numbering approximately 50 per platelet, are the predominant platelet granules.
63 They are approximately spherical in shape, with an outer membrane enclosing two distinct intragranular zones that vary in electron density. The larger, electron-dense region is often eccentrically placed and consists of a nucleoid material that is rich in platelet-specific proteins such as
β-thromboglobulin.
64 The second zone, of lower electron density, lies in the periphery adjacent to the granule membrane and contains tubular structures with adhesive GPs such as von Willebrand factor (vWF) and multimerin,
along with factor V.
65,66,67 Platelets take up plasma proteins and store them in their
α-granules.
68,69,70 Select
α-granule proteins are discussed below and listed in
Table 16.2.
Three proteins,
β-thromboglobulin, PF4, and thrombospondin, are synthesized in megakaryocytes and highly concentrated in
α-granules. The first two,
β-thromboglobulin and PF4, show homology in amino acid sequence and share the additional features of localization in the dense nucleoid of
α-granules, heparin-binding properties, and membership in the CXC family of chemokines.
14,71,72,73 and 74 Together, they constitute approximately 5% of total platelet protein, and they can serve as useful markers for platelet activation in serum or plasma.
75,76 Thrombospondin may comprise up to 20% of the total platelet protein released in response to thrombin, and likely participates in multiple biologic processes.
77,78vWF is also synthesized by megakaryocytes and is present in the tubular structures of the
α-granule peripheral zone, similar to its localization within Weibel-Palade bodies of vascular endothelial cells.
66,79 Factor V and multimerin, a factor V/Va-binding protein,
67,80 co-localize with vWF in platelets but not in endothelial cells. Fibrinogen is also found in
α-granules, but is incorporated actively from plasma and not synthesized by megakaryocytes.
69 In fact, small amounts of virtually all plasma proteins, such as albumin, immunoglobulin G (IgG), fibronectin, and
β-amyloid protein precursor, may be taken up into the platelet
α-granules.
79,81,82 and 83 α-Granules also contain many growth factors, including platelet-derived growth factor, transforming growth factor-
β1 (TGF-
β1), and vascular endothelial growth factor. These signaling molecules may contribute to the mitogenic activity of platelets.
84,85 A role for platelet
α-granule proteins in angiogenesis has been recently reported. Both pro- (e.g., VEGF) and antiangiogenesis proteins (e.g., endostatin) are stored within
α-granules. How populations of angiogenesis regulatory proteins may be selectively compartmentalized and released from platelets is an area of active investigation.
86,87,88,89 and 90 Likewise, the activation and functions of platelet-derived TGF-
β1 in cardiac fibrosis and other conditions have been described.
91,92Platelet
α-granules serve as an important reservoir for
αIIbβ3 that contributes significantly to the surface fibrinogen receptors present on activated platelets.
93,94,95 The
α-granule membrane protein, P-selectin (granule membrane protein-140) is translocated to the plasma membrane after platelet activation.
96,97 Finally, a number of additional proteins have been located to the surface of
α-granules alone, including CD9, platelet endothelial cell adhesion molecule-1 (PECAM-1), Rap 1b, GPIb-IX-V, and osteonectin
98,99 and 100 (
Table 16.2).
The platelets and megakaryocytes of patients with gray platelet syndrome have decreased numbers of
α-granules and reduced levels of some proteins. It is proposed that there is incorrect targeting of
α-granule proteins to the
α-granule in the megakaryocyte in this disease.
101,102
Dense Bodies
Dense bodies, numbering approximately five per platelet, are exceptionally electron-dense and easily distinguished by electron microscopy because of their distinctive “bull’s-eye” appearance.
103,104 With an approximate diameter of 250 nm, these granules contain a large reservoir of ADP, a critical agonist for platelet activation that amplifies the effect of other stimuli.
132 In addition to this nonmetabolic pool of ADP, the dense bodies are rich in ATP, pyrophosphate, calcium, and serotonin (5-hydroxytryptamine), with lesser amounts of guanosine triphosphate (GTP), guanosine diphosphate (GDP), and magnesium.
74 The adenine nucleotides are synthesized and segregated by megakaryocytes, whereas serotonin is incorporated into dense granules from the plasma by circulating platelets.
133,134,135 There is more ADP than ATP in dense bodies, and both can lead to adenosine monophosphate (AMP). In turn, AMP can be dephosphorylated to adenosine or cyclized to produce cyclic AMP, an inhibitor of the platelet-stimulatory response. The dense granule membrane contains P-selectin and granulophysin.
136
Lysosomes
Lysosomes are small, acidified vesicles, approximately 200 nm in diameter,
105 that contain acid hydrolases with pH optima of 3.5 to 5.5, including
β-glucuronidase, cathepsins, aryl
sulfatase,
β-hexosaminidase,
β-galactosidase, heparitinase, and
β-glycerophosphatase. Additional proteins found in lysosomes include cathepsin D and lysosome-associated membrane proteins (LAMP-1/LAMP-2), which are expressed on the plasma membrane after activation).
106,107 Lysosomal constituents are released more slowly and incompletely (maximally, 60% of the granules) than
α-granules or dense-body components after platelet stimulation, and their release also requires stronger agonists such as thrombin or collagen.
Organelles: Microperoxisomes, Coated Vesicles, Mitochondria, and Glycogen
Peroxisomes are rare, small (90 nm in diameter) granules, demonstrable with alkaline diaminobenzidine as a result of their catalase activity.
108 The structure may participate in the synthesis of platelet-activating factor.
109
Mitochondria in platelets are similar, with the exception of their smaller size, to those in other cell types. There are approximately seven per human platelet, and they serve as the site for the actions of the respiratory chain and the citric acid cycle.
110 Glycogen is found in small particles or in masses of closely associated particles, playing an essential role in platelet metabolism.
111