FIGURE 56-1. The parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptor interacts with indistinguishable efficiency and efficacy with PTH and PTHrP, and it activates at least two distinct second messenger systems, cyclic adenosine monophosphate and Ca2+/inositol 1,4,5-triphosphate. The receptor is abundantly expressed in bone and kidney, where it mediates the endocrine actions of PTH, and in the metaphyseal growth plate and numerous other tissues, where it mediates the autocrine/paracrine actions of PTHrP.
PTH and PTHrP most likely evolved from a common ancestral precursor. Despite this common evolutionary origin, both peptides share only limited overall amino acid sequence identity, yet at least their N-terminal regions are sufficiently homologous to enable them to bind to and activate a common G protein–coupled receptor, the PTH/PTHrP receptor (also referred to as PTH1R).7–9 This receptor mediates the most important biological actions of both peptides: PTH-dependent regulation of calcium and phosphorous homeostasis and PTHrP-dependent regulation of endochondral bone formation.10–14
This chapter reviews (1) the comparative chemistry of PTH and PTHrP, their genes, and their interactions with the PTH1R; (2) the current molecular models of productive interactions of the two ligands with their common receptor; and (3) the different biological roles of both peptides on target tissues, such as the role of PTH in calcium homeostasis and bone turnover, the role of PTHrP in bone and cartilage development, and the role of PTH in regulating renal phosphate excretion (see Fig. 56-1). However, the chapter does not review the potentially numerous and still incompletely characterized biological roles of PTHrP outside the field of mineral ion homeostasis and bone biology. The evolutionary history of the principal PTH/PTHrP receptor is reviewed, as well as the functional characteristics of two novel, closely related receptors and the pharmacologic and physicochemical evidence for several additional, still incompletely characterized, receptors for PTH and PTHrP.
Regulation of Mineral Ion Homeostasis—General Considerations
To ensure a multitude of essential cellular functions, the extracellular concentration of calcium (Ca2+o) is maintained within narrow limits.15,16 In terrestrial vertebrates, calcium is necessary for adequate mineralization of the skeleton, which provides mechanical support and protection for internal organs and acts as levers for the various muscle groups involved in locomotion. Because of its high calcium content, 99% of the body’s supply, the skeleton also serves as the most important reservoir from which calcium can be rapidly mobilized. Because food intake and thus the nutritional supply of calcium are usually discontinuous, intestinal calcium absorption occurs only intermittently. Maintenance of a constant blood calcium concentration thus constitutes a major homeostatic challenge, which during evolution led to the development of highly efficient mechanisms to increase intestinal calcium absorption, reduce urinary calcium losses, and facilitate, if necessary, rapid mobilization of calcium from the skeletal reservoir (see Chapter 60).16
In contrast to these environmental challenges of most terrestrial vertebrates, marine animals, which are usually exposed to the high environmental calcium concentration of seawater (10 mM) had to adopt mechanisms by which extracellular calcium could be reduced.17,18 Unlike the diet of terrestrial animals, seawater provides only a very limited supply of phosphate, and this environmental deficiency resulted in the development of mechanisms to conserve phosphate. It thus appears plausible that the efficient intestinal absorption of phosphate and the impressive capacity of the mammalian kidney to retain phosphate15,19 are remnants of earlier evolutionary adaptations to life in the low-phosphate environment of the oceans. To reduce blood calcium concentrations, fish use stanniocalcin, which is produced by the corpuscles of Stannius, as well as several other hormonal factors.17,18,20 Some data indicate that the mammalian homologue of stanniocalcin has similar properties when tested in rodents, but it remains uncertain whether this peptide hormone has a significant physiologic role in mammalian mineral ion homeostasis.21 A widely expressed mammalian peptide, stanniocalcin 2, that was discovered because of its structural homology with stanniocalcin, appears to inhibit phosphate uptake in renal epithelial cells.22 However, newer data (see later) indicate that fibroblast growth factor-23 (FGF-23) and perhaps soluble frizzled-related protein 4 (sFRP4), dentin matrix protein 1 (DMP-1), and matrix extracellular phosphoglycoprotein (MEPE)23 are more likely to be involved physiologically in the regulation of mammalian phosphate homeostasis (see Chapter 61). Calcitonin, made by the ultimobranchial bodies in fish, has a calcium-lowering function in these vertebrate species, but its biological role in mammals remains uncertain (for review, see Chapter 57).24
Parathyroid Hormone
PTH and the active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], are the principal physiologic regulators of calcium homeostasis in humans and all terrestrial vertebrates.11,25,26 Synthesis and secretion of PTH are stimulated by any decrease in blood calcium, and conversely, secretion of the hormone is inhibited by an increase in blood calcium.27–29 This rapid negative feedback regulation of PTH production, along with the biological actions of the hormone on different target tissues, represents the most important homeostatic mechanism for minute-to-minute control of calcium concentration in the extracellular fluid (ECF) (Fig. 56-2).30–32 In contrast to the rapid actions of PTH, 1,25(OH)2D3 is of critical importance for long-term, day-to-day, and week-to-week calcium balance (see Chapter 58). The actions of both hormones are coordinated, and each influences the synthesis and secretion of the other. Calcitonin, the third of the calciotropic hormones known to be important in the regulation of vertebrate mineral ion homeostasis (see Chapter 57), may be vestigial in humans with respect to calcium homeostasis and will not be discussed in this brief review of physiology.
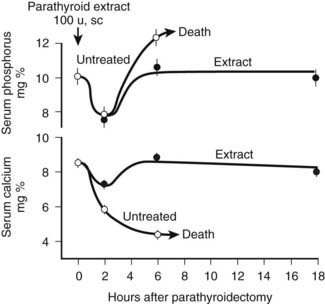
FIGURE 56-2. Rate of change in blood phosphorus and calcium levels in rats with stressed calcium homeostasis (low-calcium diet) after parathyroidectomy without treatment or with 100 U of parathyroid hormone extract given in addition at the time of parathyroidectomy. Rapid and usually fatal hypocalcemia and hyperphosphatemia result within hours unless hormone is given.
(Data from Munson PL: Studies on the role of the parathyroids in calcium and phosphorus metabolism, Ann N Y Acad Sci 60:776–796, 1955.)
At least three distinct but coordinated actions of PTH increase the flow of calcium into the ECF and thus increase the concentration of blood calcium (see Fig. 56-2).27–29 Through its rapid actions on the kidney and bone, which are all mediated through the PTH/PTHrP receptor and subsequent secondary messages in specific and highly specialized cells, PTH increases the release of calcium from bone, reduces the renal clearance of calcium, and stimulates the production of 1,25(OH)2D3 by activating the gene encoding 25-hydroxyvitamin D-1α-hydroxylase (1α-hydroxylase) in the kidney. The relative importance of the first two actions of PTH on the rapid, minute-to-minute regulation of calcium is not definitively resolved, but most physiologists have stressed the importance of the effects of PTH on bone in maintaining hour-to-hour calcium homeostasis in the ECF. Several lines of evidence, such as that provided by calcium kinetic analysis, indicate a transfer between ECF and bone of as much as 500 mg of calcium daily, which is equivalent to one-fourth to one-half the total ECF calcium content.15 Besides regulating this transfer of calcium from bone through direct breakdown of bone tissue (mineral and matrix), PTH influences the rates of exchange of calcium adsorbed to the surface of bone; this exchangeable calcium pool can be stimulated to provide a rapid and substantial rate of entry of calcium into blood. In addition to these PTH-dependent actions on bone, actions of PTH on the kidney may also be extremely important in the precise hourly regulation of ECF calcium. The third action of PTH on calcium homeostasis—namely, enhancement of intestinal calcium absorption—is indirect and involves the synthesis of 1,25(OH)2D3 from the biologically inactive precursor 25(OH)D3. However, it is difficult to quantitatively analyze or to proportionately contrast the relative physiologic importance of the direct and indirect actions of PTH on the three principal target tissues: kidney, bone, and intestine.
The complexity of bone as a tissue and the many detectable rates of exchange of calcium between the skeleton and the ECF have made the action of PTH on the skeleton difficult to analyze. The state of calcium in blood is complex; much of the calcium is present as chelates or is bound to plasma proteins (for detailed review, see Chapter 60). Because actual filtered loads depend on the ratio of free and bound forms of calcium, it is difficult to calculate renal calcium clearance accurately. The different PTH-dependent actions to promote calcium entry into the ECF are most clearly defined in conditions of deficiency or excess of PTH, such as during experiments in animals or during controlled observations in patients with disorders of parathyroid gland function. The experimental data in these extremes abundantly affirm the crucial calcium homeostatic role of PTH. However, because of continuous and rapid adjustments in mineral ion concentration, it can be difficult to observe the consequences of hormone action under normal physiologic conditions. For example, the rate of PTH secretion changes continually and rapidly so that the controlled variable, calcium, remains constant, and it may therefore be difficult to experimentally detect small corrective changes.
Teleologically, the action of PTH on the regulation of blood phosphate concentration in terrestrial species is best understood as a secondary, rather than a homeostatic, action. Phosphate is abundant in the food chain in terrestrial existence. Phosphate deficiency, unlike calcium deficiency, in the absence of specific organ dysfunction is, therefore, an unlikely environmental challenge (see Chapter 61 for detailed review of the regulation of phosphate homeostasis). To correct a deficiency in calcium, mineral stores in bone can be rapidly dissolved; such activity results, however, in the simultaneous liberation of ionic calcium and phosphate. Because a high blood phosphate level tends to lower the calcium concentration through multiple mechanisms, the rise in blood calcium that occurs after bone dissolution (desirable homeostatically) is beneficial only if the concomitant increase in blood phosphate concentration (undesirable) can be rapidly corrected. To maximize the control of calcium homeostasis, PTH thus has divergent actions on renal tubular handling of the two mineral ions: It increases the retention of calcium and at the same time diminishes reabsorption of phosphate. Through these mechanisms—namely, increased renal phosphate clearance to prevent hyperphosphatemia and increased tubular calcium reabsorption—PTH guarantees that an elevation in blood calcium results from the increased release of calcium from bone. The renal action of PTH on phosphate homeostasis is biologically predominant over the increased phosphate flux from bone. Consequently, parathyroidectomy (experimentally, in animals) or renal resistance to PTH, as in patients with pseudohypoparathyroidism or renal failure, leads not only to hypocalcemia but also to an increase in blood phosphate and a marked reduction in urinary phosphate excretion (see Fig. 56-2). This finding demonstrates the importance of the PTH-dependent action on phosphate homeostasis in the kidney, which becomes particularly important in disease states when high bone turnover is the result of dietary calcium deficiency or lack of biologically active vitamin D.
CHEMISTRY
The first extracts from bovine parathyroid glands were described in 1925, and the content of biologically active PTH was assessed by their hypercalcemic and phosphaturic properties.2,3 However, it was not until 1959, when Aurbach33 and Rasmussen and Craig34 developed improved extraction procedures, that it became possible to isolate and purify sufficient quantities to determine the primary structure of bovine, porcine, and human PTH through the protein sequence determination methods.35–40 Two groups independently determined the sequences of human and bovine hormones.35–40 Shown in Fig. 56-3A are the sequences of the bovine, porcine, and human hormones determined by one group.36,37,39,40 Discordant sequences for the human PTH polypeptide, and in one position for the bovine hormone, published by the other group,35,38 are not shown in Fig. 56-3A, since nucleotide sequence analysis of genomic and complementary DNA confirmed the amino acid sequences of the first group (the only exception was residue 76 in human PTH, which was determined to be glutamine instead of glutamic acid).36,37,40 Based on these amino acid sequences, the PTH(1–34) fragments of the different species were synthesized, and their biological activities were compared in vitro and in vivo with those of highly purified intact PTH from the same species (Table 56-1). Molecular cloning techniques then led to the deduction of the amino acid sequences of rat, chicken, and dog PTHs,41–44 followed more recently by the identification of PTH molecules in other mammals and fish, as shown in Fig. 56-3A and discussed later. The synthetic peptides used in parathyroid hormone research today are based largely on the (1-34) regions of the mammalian hormone sequences shown in Fig. 56-3A.45,46
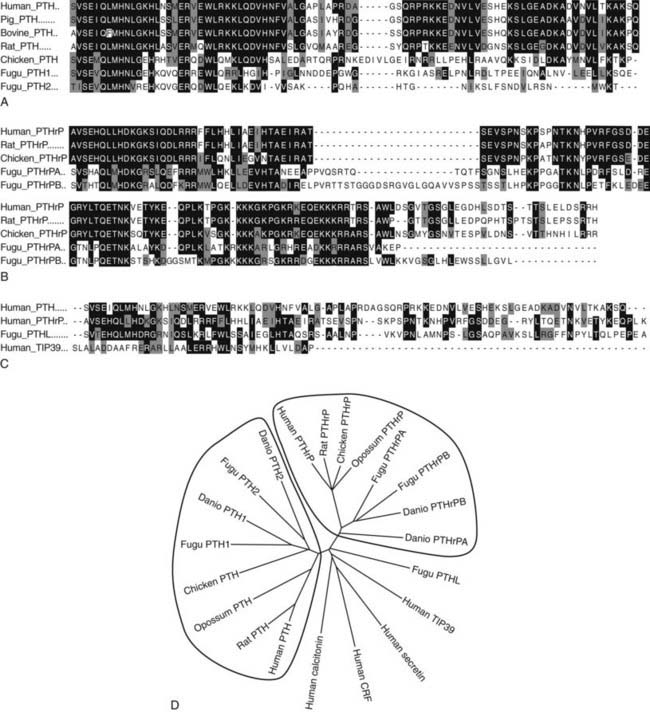
FIGURE 56-3. Sequence Relationships in PTH and PTHrP peptides. Comparisons of amino Acid sequences of PTH and PTHrP peptides from different species are shown in A and B, respectively. Comparison of human TIP39 and a PTH-Like peptide from the puffer fish (Takifugu rubripes) to human PTH and PTHrP (the (1–84) region only) is shown in C. In A-C, amino acid identities are shown on black field and similarities on gray field. A phylogenetic tree of the ligands is shown in D, with other family B receptor ligands, secretin, calcitonin, and cotricotropin-releasing factor (CRF) included as marginally homologous ligands. Fugu PTHL is speculated to be a precurssor to PTH and PTHrP.
Table 56-1. Comparison of the Biological Activity of Parathyroid Peptides from Different Species
| Peptide | Potency (MRC mmg)* | |
|---|---|---|
| In Vitro Rat Renal Adenyl Cyclase Assay | In Vivo Chick Hypercalcemia Assay | |
| Native hormones | ||
| Bovine 1-84 | 3000 (2500-4000) | 2500 (2100-4000) |
| Porcine 1-84 | 1000 (850-1250) | 4800 (3300-7000) |
| Human 1-84 | 350 (275-425) | 10,000 (9060-13,400) |
| Synthetic fragments | ||
| Bovine 1-34 | 5400 (3900-8000) | 7700 (5200-11,100) |
| Human 1-34 | 1700 (1400-2150) | 7400 (5200-9700) |
| [Ala1]-human 1-34 | 4300 (3400-5400) | — |
* Values are expressed as mean potency with 95% confidence intervals and are based on Medical Research Council research standard A for parathyroid hormone.
From Rosenblatt M, Kronenberg HM, Potts JT Jr: Parathyroid hormone. In DeGroot L (ed): Endocrinology, ed 2, Philadelphia, 1989, WB Saunders, p 853.
Extensive sequence homology is present in the mammalian PTH species (see Fig. 56-3A and D); these molecules consist of a single-chain polypeptide with 84 amino acids and a molecular weight of approximately 9400 daltons (that of human PTH[1–84] is 9425 D). The N-terminal region of PTH, which is necessary and sufficient for the regulation of mineral ion homeostasis, shows high sequence conservation among all the vertebrate species (see Fig. 56-3A). The middle portions of the different molecules exhibit the most structural variation, which could suggest that this region of PTH is only of limited functional importance. The nonmammalian PTH homologues of chicken42,43 and fish species Danio rerio (zebrafish)47 and Takifugu ruberipes (puffer fish)48,49 diverge considerably from the mammalian hormones C-terminal of amino acid residue His32. Interestingly, both fish species have two distinct genes encoding two separate PTH molecules, called PTH1 and PTH2.47,48 The zebrafish peptides are considerably shorter than mammalian PTH (67 and 68 residues), while fugu PTH1 is predicted to be 81 residues in length and fugu PTH2 is predicted to be 63 residues.48
After the original work establishing that the first 34 amino acids of mammalian PTH were sufficient to produce a fully active synthetic peptide,45,46 much work has centered on defining the minimum pharmacophore essential for biological activity. In a following section, we describe how sites of ligand interaction with the PTH/PTHrP receptor were defined by performing assays with products of various combinations of shortened and modified PTH ligands and mutagenized receptors. Furthermore, a fusion protein consisting of ligand substituted for most of the receptor amino-terminal extracellular domain was generated, and this helped define a much smaller minimum chain length of PTH peptide needed for biological activity.50 As also discussed later in the section on hormone/receptor interactions, it has been determined that substitutions of non-naturally occurring amino acids (e.g., α-aminoisobutyric acid at positions 1 and 3 in the primary ligand structure) favoring formation of an α-helix, even in short peptides, such as PTH(1–14), produce peptides that are highly potent when tested in vitro using cell-based assays and have highly stabilized helical structure in solution51–55(Fig. 56-4).
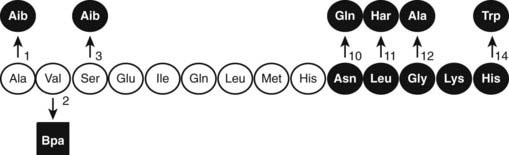
FIGURE 56-4. The native (rat) PTH(1-14) sequence and activity-modifying substitutions. The six substitutions shown above the sequence, when combined, enhance activity by as much as 100,000-fold; the Bpa2 substitution shown below confers antagonist properties to the peptide. The (1-9) region (nonshaded circles) is thought to be the minimum-length agonist pharmacophore. Nonencoded amino acids include: α-amino-isobutyric acid (Aib), homoarginine (Har), and parabenzoyl-l-phenylalanine (Bpa).
The in vitro activity of the native PTH(1-14), which is quite weak, is improved about 100,000-fold by the modifications indicated in Fig. 56-4. Shorter PTH peptides have also been shown to be active in vivo, since some cause hypercalcemia and are anabolic on bone, although their potency is much less than that of PTH(1-34) due to a more rapid clearance.56 Replacement of valine-2 in these peptides with bulky amino acids, such as tryptophan or parabenzoyl-l-phenylalanine (Bpa), results in competitive antagonist peptides defective for AC/cAMP and PLC/IP3/Ca2+ signaling, thus confirming the critical role that this conserved valine plays in receptor activation.57 Other longer-length PTH or PTHrP analogs having residue-1 (serine or alanine) replaced by glycine,58 Bpa,59 or tryptophan60 exhibit signal-selective properties in that they efficiently stimulate the cAMP cellular pathway but not the inositol tri-phosphate/intracellular Ca2+ pathway.
The three-dimensional structures of intact PTH(1-84) and the N-terminal biologically active fragments of both PTH and PTHrP have been analyzed by various solution-based methods, including nuclear magnetic resonance (NMR) spectroscopy. Interpretation of these results in terms of biological mode of action is not straightforward, because the ligand interacts with a membrane-embedded receptor, and the biophysical properties of this environment, as well as the receptor-induced conformational changes that occur, are mostly unknown. However, the bioactive (1-34) portions of the ligands are generally found to contain helical structure within their N-terminal and especially C-terminal portions, with some flexibility in the midregion.61–64 An x-ray crystallographic study of PTH(1-34) revealed α-helical structure extending nearly the full length of the peptide.65 A persisting question is thus whether the ligand bound to the receptor adopts a linear and extended62 or “U-shaped” folded structure, the latter suggested by tertiary interactions seen in some solution-phase biophysical studies.61,63,64 Of particular interest has been the question of whether common structural features would be discerned for PTH and PTHrP that could explain their use of apparently overlapping binding sites on the common PTH/PTHrP receptor; to some extent, the helical propensities of at least the C-terminal domains of the peptides are consistent with this possibility.62,66,67
Therefore, consistent with their rather limited homology in primary structure (see Fig. 56-3), convincing evidence has not yet been provided for the conclusion that the N-terminal fragments of PTH and PTHrP display a similar secondary structure in solution. Because of their generally demonstrated similar potencies at the PTH/PTHrP receptor, it seemed likely that both ligands would adopt very similar conformations when part of the active hormone-receptor complex. However, recent data (discussed later) suggest that each ligand selectively binds to or induces a distinct receptor confirmation. Ideally, each hormone should be co-crystallized with the PTH/PTHrP receptor to permit analysis by x-ray diffraction of those intermolecular interactions that are characteristic of the biologically active hormone-receptor complexes. G protein–coupled receptors such as the PTH/PTHrP receptor have multiple membrane-embedded domains and are likely to have complex three-dimensional structures. Interaction with either PTH or PTHrP appears to involve several distinct receptor domains (see later discussion) that may undergo significant conformational changes after ligand binding has occurred, which makes it even more challenging to conduct x-ray or multidimensional NMR analyses. Recent advances, however, have been made it possible to co-crystallize the extracellular portion of the PTH/PTHrP receptor with the carboxyl end of the PTH (1-34) peptide68 and to crystallize the membrane-spanning portion of a distantly related GPCR, the β2-adrenergic receptor.69
EVOLUTION
To maintain extracellular calcium and phosphate concentrations within narrow limits, the intricate regulatory system outlined, in which PTH plays the most important role, developed in the terrestrial animals. In mammals, PTH is produced almost exclusively by the parathyroid glands (only small amounts of its messenger RNA [mRNA] have been detected elsewhere70,71). During evolution, these glands first appear as discrete organs in amphibians, that is, with the migration of vertebrates from an aquatic to a terrestrial existence, and their appearance most likely represents an evolutionary adaptation to an environment that is, by comparison to seawater, low in calcium.17,18,72 Parathyroid glands have not been identified in fish or invertebrate species, but earlier immunologic and RNA hybridization data from fish provided evidence for expression of PTH proteins in several tissues, including pituitary,17,18,73 plasma, brain, kidney, spinal cord, ultimobranchial gland, as well as in the ventral neural tube and mineralizing jaw during development.48,74 Now, with the rapid advances in characterization of complete genomes of multiple species, we have definitive proof of the earlier evolutionary origin of both PTH and PTHrP (see Fig. 56-3). Gene analyses of several teleost fish species, including the zebrafish, Danio rerio, and the puffer fishes Takifugu rubripes and Tetraodon fluviatilis, reveal the duplication of the PTH gene in each case.48,75 Both the PTH1 and PTH2 peptides derived from the zebrafish activate the PTH/PTHrP receptors from different species,49,75 and indeed, a fugu PTH(1-34) peptide has been shown to induce bone anabolic effects in osteopenic ovariectomized rats.76
In addition to PTH, the teleost fish also express PTHrP, again encoded by duplicate genes.75,77–80 Furthermore, PTHrP immunoreactivity has been detected in the cartilaginous sharks and rays81 and in a more primitive agnathan, the lamprey.82 The teleost PTHrPs contain some amino acid residues characteristic of mammalian PTH; for example, fish PTHrP contains Met at position 8, Trp at position 23, and Leu at position 28, which are amino acid residues found in mammalian PTH. However, there is only one amino acid residue, Gln(Q)25, in fish PTH that is found in some mammalian PTHrP species but not in mammalian PTH (see Fig. 56-3). This pattern suggests that the fish proteins may be phylogenetically closer to a common PTH/PTHrP precursor than are the mammalian proteins (see later). Indeed, in addition to duplicate copies of PTH and PTHrP genes, the puffer fish genome contains a fifth gene that encodes a protein containing amino acid residues characteristic of both PTH and PTHrP. This gene, called PTH-L, is phylogenetically an intermediary to PTH and PTHrP and may thus represent first definitive evidence for an ancestral gene from which the two divergent ligand forms evolved (see Fig. 56-3C and D).48,75
THE PTH GENE AND ITS mRNA
The human PTH gene consists of three exons located on chromosome 11p15.83–86 The first exon is 85 nucleotides in length and is noncoding (Fig. 56-5). Exon 2 (90 bp) encodes most amino acids of the prepropeptide sequence, whereas the third exon (612 bp) encodes the remainder of the propeptide sequence and all amino acids of the mature peptide, and it constitutes the 3′ noncoding region.87 Several frequent intragenic polymorphisms (TaqI and PstI88; BstBI89; DraIII90; XmnI91), and a tetranucleotide repeat ([AAAT]n;92) have been identified in the human PTH gene, and some were shown to be informative in genetic linkage studies.93–95 Two mRNAs that are 822 and 793 bp in length are derived in the human gene from the two transcriptional start sites, which follow two different functional TATA boxes that are separated by 29 bp.87 Two closely spaced TATA boxes and two distinct transcripts are also derived from the bovine PTH gene, while rat and chicken PTH genes give rise to only one transcript; as a consequence of a long 3′ noncoding region, the transcript from the chicken PTH gene is unusually long and comprises 2.3 kb.25,96 The genes encoding zebrafish PTH1 and PTH2 have a similar overall organization as the mammalian PTH genes.47
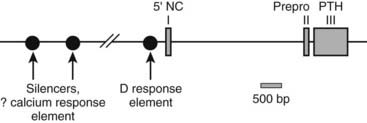
FIGURE 56-5. Schematic of the parathyroid hormone (PTH) gene along several thousand base pairs (approximate length shown by the scale marker for 500 bp). The three exons in the mRNA are represented as numbered rectangles. Control elements are identified in the 5′ noncoding region (5′ NC). A region responsive to vitamin D is within a few hundred base pairs of exon 1. Far upstream are silencers involved in calcium regulation.
PTH BIOSYNTHESIS AND INTRAGLANDULAR PROCESSING
During the synthesis of the preproPTH molecule, the signal sequence, which comprises the 25-amino-acid-containing “pre” sequence, is cleaved off after entry of the nascent peptide chain into the intracisternal space bounded by the endoplasmic reticulum. A heterozygous mutation in this leader sequence, which changes a cysteine to an arginine at position −8 and thus impairs processing of preproPTH to proPTH, has been identified as the most plausible molecular cause of an autosomal dominant familial form of hypoparathyroidism.97,98 The mutant hormone was found to be trapped intracellularly, predominantly in the endoplasmic reticulum (ER), leading to a marked up-regulation of ER stress-responsive proteins (BiP and PERK) and the proapoptotic transcription factor, CHOP, indicating that apoptosis-mediated parathyroid cell death is the likely cause of the observed hypoparathyroidism.99
Subsequent to the removal of the pre-sequence, the pro-peptide is transported to the trans-Golgi network, where the pro-sequence (amino acid residues −6 through −1) is removed.100 This latter process may involve furin (paired basic amino acid cleaving enzyme) and/or proprotein convertase-7 (PC-7), which are both expressed in parathyroid tissue; their expression levels do not appear to be regulated by either calcium or 1,25(OH)2D3.101,102 After removal of the basic pro-sequence, the mature polypeptide, PTH(1-84), is packaged into secretory granules. Two proteases, cathepsins B and H, are subsequently involved in the intraglandular generation of carboxyl-terminal PTH fragments from the intact hormone; no amino-terminal PTH fragments appear to be released from the gland.103–105 Since small or intermediate-size carboxyl-terminal fragments of PTH are unlikely to be involved in the regulation of calcium homeostasis, the intraglandular degradation of intact PTH is thought to represent an inactivating pathway, at least with regard to the regulation of mineral ion homeostasis. Consistent with this conclusion, hypercalcemia results in a substantial decrease in PTH secretion and, furthermore, favors the secretion of carboxyl-terminal PTH fragments, including a previously undetected large molecular species that are truncated at the amino-terminus (see following section).105–108 However, recent studies have shown that some amino-terminally truncated PTH fragments, such as PTH(7-84), have hypocalcemic properties in vivo and can furthermore reduce the formation of osteoclasts in vitro.109
The pool of stored, intracellular PTH is small, and the parathyroid cell must therefore have mechanisms to increase hormone synthesis and release in response to sustained hypocalcemia. One such adaptive mechanism is to reduce the intracellular degradation of the hormone, thereby increasing the net amount of intact, biologically active PTH that is available for secretion. During hypocalcemia, the bulk of the hormone that is released from the parathyroid cell is intact PTH(1-84).103–105,107,108 As the level of Ca2+o increases, a greater fraction of intracellular PTH is degraded, and with overt hypercalcemia, most of the secreted immunoreactive PTH consists of biologically inactive C-terminal fragments.10,25,26
REGULATION OF PTH GENE EXPRESSION
Another adaptive mechanism of the parathyroid cell to sustained reductions in Ca2+o is to increase cellular levels of PTH mRNA, a response that takes several hours. A reduction in Ca2+o increases, whereas an elevation in Ca2+o reduces the cellular levels of PTH mRNA by affecting both its stability and the transcriptional rate of its gene.11,26,110,111 Available data suggest that phosphate ions also regulate, directly or indirectly, PTH gene expression. In the rat, hypophosphatemia and hyperphosphatemia, respectively, lower and raise the levels of mRNA for PTH through a mechanism that is independent of changes in Ca2+o or 1,25(OH)2D3. An elevated extracellular phosphate concentration could thus contribute importantly to the secondary hyperparathyroidism frequently encountered in patients with end-stage renal failure, who often have chronically elevated serum phosphate concentrations.
Metabolites of vitamin D, principally 1,25(OH)2D3, also play an important role in the long-term regulation of parathyroid function and may act at several levels: by affecting the secretion of PTH and regulation of its gene, by regulating transcriptional activity of the genes encoding the calcium-sensing receptor (CaSR; see later) and the vitamin D receptor (VDR), as well as by regulating parathyroid cellular proliferation.11,26,110,112 1,25(OH)2D3 is by far the most important vitamin D metabolite that modulates parathyroid function. It acts through a nuclear receptor, the VDR, often in concert with other such receptors (i.e., those for retinoic acid or glucocorticoids), on DNA sequences upstream from the PTH gene (see Chapter 58).113,114 1,25(OH)2D3-induced upregulation of VDR and CaSR expression in the parathyroid could potentiate its inhibitory action(s) on PTH synthesis and secretion.11,26,110 Noncalcemic or less calcemic analogs of 1,25(OH)2D3 inhibit PTH secretion while producing relatively little stimulation of intestinal calcium absorption and bone resorption115–117 and may thus be attractive candidates for treating the hyperparathyroidism of chronic renal insufficiency.
Adjustment of the rate of parathyroid cellular proliferation is the third adaptive mechanism contributing to changes in the overall secretory activity of the parathyroid gland. Under normal conditions, parathyroid cells have little or no proliferative activity. The parathyroid glands, however, can enlarge greatly during states of chronic hypocalcemia, particularly in the setting of renal failure, probably because of a combination of hypocalcemia, hyperphosphatemia, and low levels of 1,25(OH)2D3 in the latter condition.
REGULATION OF PARATHYROID HORMONE SECRETION
A large number of factors modulate PTH secretion in vitro,11,26,118 but most of these factors are not thought to control hormonal secretion in vivo in a biologically relevant manner. Therefore, we focus in this section principally on factors that are the most physiologically meaningful regulators of PTH secretion—that is, the extracellular ionized calcium concentration itself (Ca2+o), 1,25(OH)2D3, and the level of extracellular phosphate ions. Of these three, Ca2+o is most important in the minute-to-minute control of PTH secretion. Indeed, the actions of 1,25(OH)2D3 and phosphate ions on the secretion of PTH probably result at least in part from their effects on hormonal biosynthesis rather than secretion per se.11,26,118 Ca2+o also modulates several other aspects of parathyroid function that indirectly affect PTH secretion, including PTH gene expression, the hormone’s intracellular degradation, and parathyroid cellular proliferation, as described previously. Recent data have shown that novel factors playing key roles in phosphate homeostasis, especially FGF-23 and α-klotho (a coreceptor for FGF receptors), also modulate parathyroid function, inhibiting119,120 and enhancing121 parathyroid function, respectively. Our rapidly improving understanding of how these factors participate in phosphate homeostasis is described in detail in Chapter 61.
Physiologic Control of PTH Secretion by Ca2+o
As illustrated in Fig. 56-6A, the relationship between PTH and Ca2+o is represented by a steep inverse sigmoidal curve that can be quantitatively described by four parameters.122–124 These are the maximal rate of PTH secretion at low Ca2+o (parameter A); the slope of the curve at its midpoint (parameter B); the value of Ca2+o at the midpoint (e.g., the “set point” or the level of Ca2+o half-maximally suppressing PTH release; parameter C); and the minimal secretory rate at high Ca2+o (parameter D) (see Fig. 56-6B). Parameter A in vivo is the sum of the maximal rates of PTH release from all individual parathyroid chief cells, as reflected by the resultant, maximally stimulated level of circulating PTH. Because of the steepness of the Ca2+o-PTH relationship, small alterations in Ca2+o evoke large changes in PTH release, thereby contributing importantly to the near constancy of Ca2+o in vivo. Indeed, parathyroid cells can readily detect reductions in Ca2+o of a few percentage points,123 and the percent coefficient of variation in Ca2+o in humans is less than 2%.125 The set point of the parathyroid gland is the key determinant of the level at which Ca2+o is “set” in vivo, although the parathyroid set point is usually slightly lower than the ambient blood Ca2+.126 Thus, the parathyroid cell is normally more than half-maximally suppressed at normal levels of Ca2+o and has a large secretory reserve for responding to hypocalcemic stress. Nevertheless, PTH levels in vivo also fall dramatically (e.g., by 80%) when Ca2+o rises to frankly hypercalcemic levels,122,123 which is thought to contribute importantly to the mineral ion homeostatic system’s defense against hypercalcemia.126 Furthermore, elevating Ca2+o also decreases the proportion of secreted intact PTH because of increased intraglandular degradation to inactive fragments (see the earlier section, PTH Biosynthesis and Intraglandular Processing, and the later section, Metabolism of PTH).106,127 Even with severe hypercalcemia, however, some residual release of intact PTH(1-84) still occurs in vivo and persists at a level approximately 5% of that observed with a maximal hypocalcemic stimulus29,108,128 (see Fig. 56-6A). This nonsuppressible basal component of PTH release may contribute to the hypercalcemia caused by hyperparathyroidism when the mass of abnormal parathyroid tissue is very great (e.g., in patients with renal failure).124,129–131
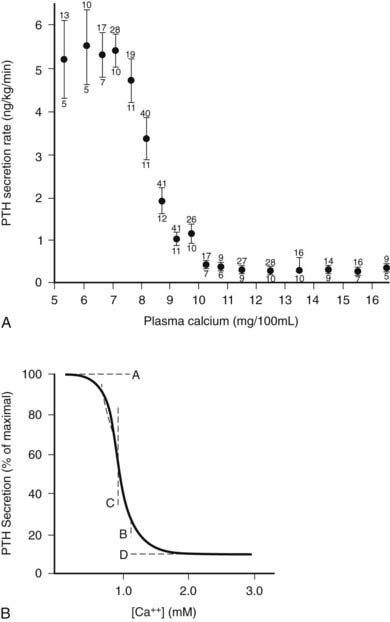
FIGURE 56-6. Inverse sigmoidal relationship between Ca2+ and parathyroid hormone (PTH) release and the four-parameter model describing these curves. A, Secretory response of bovine parathyroid glands to induced alterations in plasma calcium concentration. Calves were infused with calcium or ethylenediaminetetraacetic acid, and PTH secretion was assessed by measuring PTH levels in the parathyroid venous effluent. The symbols and vertical bars indicate the secretory rate (mean ± SE) in calcium concentration ranges of 1 or 0.5 mg/100 mL. The number of calves and samples are indicated, respectively, by numbers below and above. B, Sigmoidal curve generated by the equation Y = [(A − D)/(1 + (X/CB)] + D; the significance of A, B, C, and D are described in the text.
(A, Data from Hurst JG: Sigmoidal relationship between parathyroid hormone secretion rate and plasma calcium concentration in calves. Endocrinology 10:10, 1978; B, Data from Brown EM: PTH secretion in vivo and in vitro, Miner Electrolyte Metab 8:130–150, 1982.)
The parathyroid cell has a temporal hierarchy of responses to low Ca2+o that permits it to secrete progressively larger amounts of hormone during prolonged hypocalcemia.11,26,118 To meet acute hypocalcemic challenges, PTH is released within seconds from preformed secretory vesicles by exocytosis as dictated by the sigmoidal curve (see Fig. 56-6). Sufficient PTH is stored in the parathyroid chief cell to sustain maximal, low Ca2+o-stimulated PTH release for about 60 to 90 minutes.126 Another rapid response of the parathyroid cell to hypocalcemia that enhances its net synthetic rate of PTH is reduced intracellular hormonal degradation—the opposite of what occurs at high levels of Ca2+o—which occurs within minutes to an hour.106,127 Hypocalcemia persisting for hours to days elicits increased PTH gene expression, whereas that lasting for days to weeks or longer stimulates parathyroid cellular proliferation.11,26,118,132 A greater secretory capacity for PTH on a per-cell basis (e.g., as a result of enhanced PTH gene expression) increases maximal hormonal secretion in vivo, as does an increase in cell number as a result of parathyroid cellular proliferation (see Fig. 56-6). In severe secondary hyperparathyroidism, very large increases in parathyroid cellular mass can elevate circulating PTH levels by 100-fold or more.
In addition to responding to changes in Ca2+o per se, the parathyroid cell also appears to sense the rate of change in Ca2+o such that rapid decrements in calcium promote more vigorous secretory responses than do changes of a similar magnitude occurring more slowly.133 Furthermore, during dynamic testing of parathyroid function in vivo by induced increases or decreases in Ca2+o, PTH in blood is higher at a given serum calcium concentration when Ca2+o is falling than when it is rising (e.g., hysteresis is occurring in this relationship).134,135 The latter results in an apparent direction dependence of the secretory response, which when combined with the rate dependence just described, may allow for a physiologically appropriate, more vigorous secretory response to large rapid decrements in Ca2+o. Also present are circadian136 (for review, see Diaz et al.26) and more rapid (i.e., occurring at rates of one to six pulses per hour) phasic changes in circulating PTH levels,26,137 but the physiologic significance of these changes is not known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree