The importance of leukocytes in the defense of the organism is well known. Basic to their roles are cell multiplication, maturation, storage, and delivery to the tissues and sites of infection or cell damage. These processes are called leukocyte kinetics and are different for each leukocyte type. To simplify the discussion, each type of leukocyte is considered as a separate system, but these systems constantly interact and complement one another in the defense of the body.
Production in the Mitotic Compartment
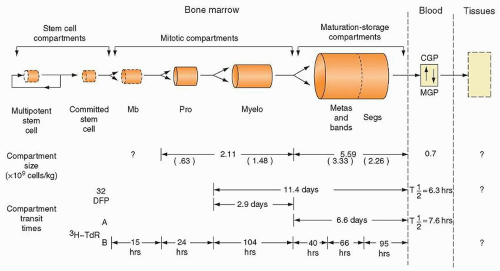
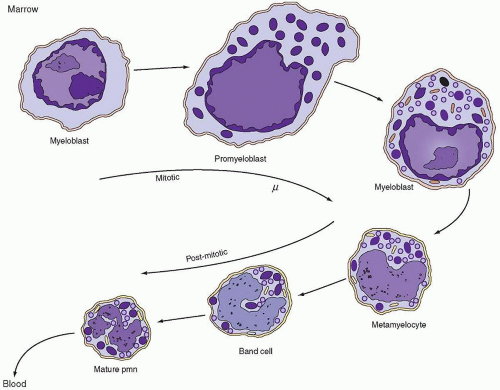
Because myeloblasts, promyelocytes, and myelocytes constitute approximately 0.9%, 3.3%, and 12.7% of the marrow cells, respectively, it has been assumed that the system has four or five divisions.
180 From a review of blood neutrophil radioactivity curves obtained after di-isopropylfluorophosphate (DF
32P) injection into humans, investigators raised the possibility of at least three divisions at the myelocyte stage.
180 Another suggestion generated from marrow differential counts was that only four or five divisions occur in the entire neutrophil proliferation scheme.
180 This supposition agreed with results from experiments in dogs
188 and with data from model studies.
189 In contrast, studies of myeloid islands in the rat thymus provided evidence for seven divisions during granulocytopoiesis: one in the myeloblast stage, two in the promyelocyte stage, three in the myelocyte stage, and a final one in the metamyelocyte stage.
134,190Calculations made from mitotic index (MI) data
172,191,192 and 193 provide estimates of cell generation time (
tg) and pool turnover time. MI
191,192,194 is defined as
MI = Nm/N
in which MI is the mitotic index for any morphologic cell pool, Nm is the number of mitoses in that pool, and N is the total number of cells in the pool. MI can also be expressed as the ratio of the time spent in mitosis (tm) to the cell generation time (tg): MI = tm/tg
By combining both definitions,
MI = Nm/N = tm/tg
By providing determined values for MI and mitotic time (tm) in the last equation, the generation time (tg) for a particular cell pool can be approximated. From tg and the pool size (N), the birth rate, Kb, can be obtained if all cells in the pool are in cycle, because each mitosis gives rise to one new cell:
Kb = N/tg
In effect, the cell birth rate is equal to the number of mitoses occurring per unit time (t), or
Kb = Nm/t
Although the concept is simple, several problems arise.
193,195 A major problem is that the morphologic boundaries of most cell pools are not clearly delineated in terms of the cell cycle.
195 For example, to calculate cell production in the myelocyte pool, it must be assumed that all myelocytes are destined to divide; that is, no cells are recognized as myelocytes that are not going to divide again. Because the daughter cells of the last myelocyte mitosis almost certainly do not suddenly become metamyelocytes on completion of division,
N in the preceding equation will be erroneously large, and thus estimates of
tg will be erroneously long. If the fraction of nonmitotic cells in the myelocyte population
were known, the calculations could be corrected for this error, as has been attempted.
170 A second major problem is the fact that values for the MI have varied considerably.
191,193,196,197,198,199 and 200 In addition, a considerable diurnal variation exists in the MI in humans, as well as in animals.
193,199,200,201 and 202Finally, to calculate the absolute neutrophil production rate (in cells per unit of time), the size of the marrow mitotic compartment must be known. Methods for measuring the sizes of marrow myeloid pools have been developed
203,204,205 and 206 (
see “Size of Marrow Compartments and Their Morphologic Subdivisions,” later in the chapter), but to date no one has measured these sizes and MI in the same animal at the same time and then calculated neutrophil production rate. Nevertheless, values for the MI for each of the neutrophil precursors capable of mitosis have been determined,
193,200 and within the assumptions inherent in such calculations,
195 neutrophil production has been estimated.
176,192Similar calculations of neutrophil production can be made from
3H-TdR-labeling index data. After flash labeling with
3H-TdR, autoradiographs of the bone marrow are obtained, and the proportion of nucleated cells that have incorporated the label into their nuclei is determined.
171,177 This labeling index (LI) represents the ratio of labeled cells, *
N, or cells in DNA synthesis (
Ns) to total cells (
N) of a defined morphologic type:
LI = *N/N = Ns/N
The LI can also be defined in terms of DNA synthesis time (
ts) and the cell generation time (
tg), because
3H-TdR is taken into the cell only during the period of DNA synthesis; thus,
LI = ts/tg
By combining both definitions,
LI = Ns/N = ts/tg
and from determined values for LI and tg, the generation time and turnover of a given cell population can be estimated. As with MI data, birth rate is a function of the population turnover time, which can be approximated from the generation time or time spent in various phases of the cell cycle:
Kb = N/tg = Ns/ts
Some of the same problems arise with the
3H-TdR LI that are encountered in the use of the MI.
195,207 In addition, the use of labeled compounds raises questions of label reuse
208,209 or elution
207 and perturbation of the cell population by the compound
168,188,210,211 and 212 or by its radioactivity.
207,213,214The LI reported for humans is myeloblast, 0.85; promyelocyte, 0.65; and myelocyte, 0.33.
171 Somewhat different values have been reported in dogs
177 and rats.
200 By using the LI for humans and a value for
ts of 5 hours (based on studies in dogs) and by determining relative compartment sizes for each cell type from the bone marrow differential count, the relative birth rates (
Kb) of cells have been calculated.
170,200Some authors have found good agreement between neutrophil production as calculated from the MI and the LI,
200 but considerable discrepancy has been reported by other authors.
170 This difference may result from the fact that the MI values obtained were low, the studies were done in different subjects at different times, and too small a value for
ts was used in the calculations.
The turnover time of a labeled compartment and neutrophil production rate also may be estimated by measuring the grain count halving time. The generation time is derived only if each cell in a given class divides and if no label feeds into the compartment from a labeled precursor class or as a result of label reuse.
215 If any of these criteria are not met, the half-time for grain count decrease is longer than the true value, and the estimate of generation time is only a maximal value. Additional disadvantages of this method are that at least several bone marrow samples distributed
throughout several half-times are needed, and that grain counting is extraordinarily tedious and subject to considerable error. Nevertheless, estimates of compartment turnover time have been made with this method by using
3H-TdR and radiosulfate.
122After flash labeling with
3H-TdR, the cohort of cells labeled during DNA synthesis may be followed as it subsequently enters mitosis, and the time course of labeled mitoses can be recorded.
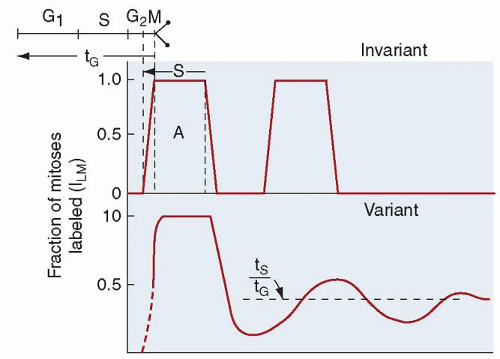
215,216 Theoretically, such curves should permit measurement of the post-DNA synthesis gap (G2), mitotic time (
tm), DNA synthesis time (
ts), cell generation time (t
g), and pre-DNA synthesis gap (G1) (
see Chapter 5 and
Fig. 7.10). In actual practice, biologic variation rounds off the percentage of labeled mitosis curves, and rapid damping of the waves of cells passing through mitosis (
Fig. 7.10) renders such measurements less precise than ideal. However, estimates of myeloid DNA synthesis time obtained with this method are approximately 11 to 13 hours in humans
215,216 and are in good agreement with estimates made in gastrointestinal mucosal cells. From the level of the damped plateau reached after a few hours, the ratio of
ts to
tg can be obtained (
Fig. 7.10), and the generation time can then be calculated. If the generation time and compartment transit time are presumed to be the same or if the proportion of cells in a compartment that is actively proliferating is known, the neutrophil production rate can be calculated.
Neutrophil Production as Measured by Cell Flow in Other Compartments
Another method for approximating neutrophil production involves following the appearance of
3H-TdR-labeled cells in the metamyelocyte compartment. Because metamyelocytes do not divide or take up
3H-TdR, the appearance of labeled cells in this compartment should reflect the flow of cells into it from the myelocyte compartment; in the steady state, this influx of cells should also reflect the turnover of the metamyelocyte compartment and thus cell production. Approximately 3 hours pass after the injection of
3H-TdR before the label appears in metamyelocytes both in dogs
177 and in humans
215; this time interval is the minimum time for myelocytes taking up the label to pass through G2 and mitosis and become metamyelocytes. After this lag, the rate of labeled cell inflow into the metamyelocyte compartment is approximately 3% to 5%/hour in both species. In the dog, cell inflow into the metamyelocyte compartment measured in this fashion is less than 50% of that calculated from LI data,
177 suggesting the existence of a major component of ineffective granulocytopoiesis, a myelocyte sink, in the normal animal. However, similar calculations in humans do not confirm the findings of such studies.
170 The resolution of this enigma requires the simultaneous measurement of cell production by using several methods in the same animal at the same time.
Similarly, the marrow production of neutrophils has been estimated from the size of the postmitotic maturation storage compartment and the compartment turnover time.
200,204,217 The compartment size is calculated from the marrow neutrophil-toerythroid (NE) ratio (determined in marrow sections) and the mean normal marrow normoblast pool size (calculated by multiplying the ratio of subjects’ erythroid iron turnover value over the mean normal value for this determination by the mean normal erythroblast population).
204 The compartment transit time is estimated by injecting
3H-TdR and noting the time required for labeled neutrophils to appear in the blood. By this method, marrow neutrophil production in humans has been calculated to be 0.85 × 10
9 cells/kg/day in the normal steady state.
Neutrophil production also can be approximated by measuring the flow of cells through the blood, the blood granulocyte turnover rate (GTR). DF
32P binds irreversibly with a number of esterase enzymes and has been shown to label neutrophils primarily.
218,219 By means of this agent, a subject’s own cells can be labeled, and the total blood granulocyte pool (TBGP) and the rate of disappearance of labeled neutrophils from the blood can be determined.
175 Similar measurements can be made by transfusing
3H-TdR-labeled cells from a suitable donor.
204 Because neutrophils leave the blood in a random manner (exponential disappearance curve), the GTR is calculated as follows from the TBGP and the
t1/2:
GTR = 0.693/t1/2 TBGP
in which 0.693 is the natural logarithm of 2 and t1/2 is the blood neutrophil half-disappearance time.
All of these methods assume that the system is in a steady state during the entire course of the measurements. If neutrophil death in the marrow is not significant, the blood GTR equals total neutrophil production. If neutrophil death in the bone marrow is significant, the blood GTR measures effective neutrophil production, and the difference between this determination and total neutrophil production is ineffective granulocytopoiesis. Measurements of neutrophil production by compartment turnover methods have given values ranging from 62 to 400 × 10
7 neutrophils/kg/day in humans (
Table 7.5)
204,220 and 150 to 560 × 10
7 neutrophils/kg/day in dogs.
204,217,221Of the methods for assessing neutrophil production just described, only the measurement of blood neutrophil turnover rate with DF
32P or
3H-TdR can be performed easily enough to be of use in studying groups of patients in a clinical setting, and even this is possible in only a few research centers.