Transplantation from Related Donors
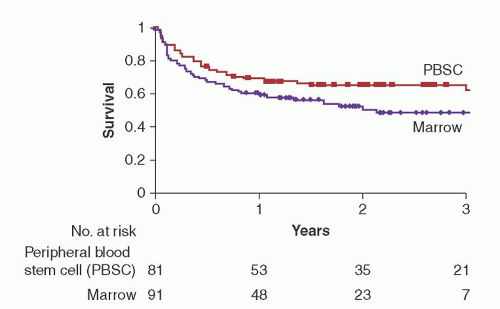
Hematopoietic cell grafts from related donors may be HLA-identical or HLA-haploidentical. The preferred allogeneic donor has been a genotypically HLA-identical sibling. A genetic match at the HLA loci between siblings is confirmed by the genotyping of 5 HLA loci including HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1. If an HLA-identical sibling is available, patients with hematologic malignancies should be transplanted with peripheral blood stem cells rather than marrow. Two phase 3 studies have now shown an improved disease-free and overall survival with transplantation of peripheral blood stem cells (
Fig. 102.2).
84,
85 Ten year follow-up of the study by Bensinger et al. showed that the benefit persisted for disease-free survival, but the likelihood of overall survival was not significantly different between the 2 groups.
86 The 10-year cumulative incidence of chronic GVHD and the duration of systemic immunosuppression were similar between the 2 groups. A third study concluded that peripheral blood was an equivalent source of HSCs compared with marrow if administered to patients with standard-risk leukemia, since a significant difference in survival could not be demonstrated.
87 All 3 studies showed an accelerated recovery of neutrophil counts in the group receiving peripheral blood stem cells. Although most studies have not shown a significant increase in the incidence of acute GVHD in the peripheral blood stem cell group, the incidence of chronic GVHD was significantly greater.
84,
85,
87,
88 Higher doses of CD34-positive cells in the peripheral blood stem cell graft (>8.0 × 10
6/kg) have been significantly associated with the development of chronic GVHD.
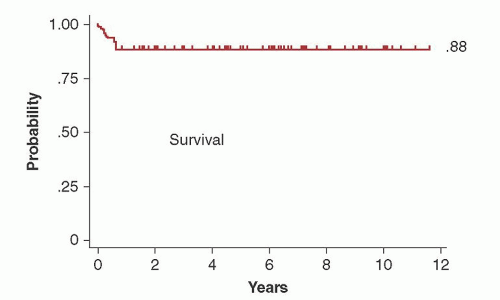
89 Since outcome is unlikely to be improved if there is an increased risk of chronic GVHD from the use of peripheral blood stem cells, patients with nonmalignant disorders are still being transplanted with marrow in some centers. With a median follow-up of 6 years, survival of aplastic anemia patients transplanted with marrow was 88% (
Fig. 102.3).
90 In a retrospective analysis by CIBMTR, rates of chronic GVHD and overall mortality were greater after transplantation with peripheral blood stem cells than marrow, especially in the younger patient population (<20 years of age).
91 Similar survivals to those observed after HCT for aplastic anemia have been reported in patients transplanted with marrow for hemoglobinopathies.
72,
92An HLA-haploidentical donor (parent, sibling, child) is available for almost all patients, but haplotype differences (genotypically identical at one HLA haplotype but nonidentical at the other HLA haplotype) are associated with a high risk of severe GVHD, graft rejection, and increased mortality with conventional posttransplant immunosuppression. However, transplants for hematologic malignancies from HLA-haploidentical family members mismatched for only one HLA antigen in the nonidentical HLA haplotype may have a similar overall survival to transplants from HLA-identical siblings.
93 In this situation, although the higher incidence of GVHD results in an increased transplant-related mortality, relapse is less frequent, resulting in no overall difference in long-term survival compared to transplants from HLA-matched siblings. Patients transplanted from HLA-haploidentical family members mismatched for 2 or more HLA loci had significantly lower overall survivals compared to patients transplanted from phenotypically HLA-matched unrelated donors.
94 In patients with advanced leukemia, transplantation with high-dose CD34-positive cell grafts which had been highly T cell-depleted resulted in 12 of 43 patients being alive and disease-free at 18 months.
95 Immune reconstitution after transplantation was poor, however.
96 High-dose cyclophosphamide (CY) early after HCT from an HLA-haploidentical donor appears effective in preventing the development of severe acute and chronic GVHD.
97,
98 In a report of parallel phase 2 studies, transplantation of HLA-haploidentical marrow grafts appeared to have favorable outcomes at 1 year compared to transplantation of umbilical cord blood grafts.
99 Patients who lack a closely matched family donor should be offered a phenotypically HLA-matched unrelated donor before considering a transplant from an HLA-haploidentical donor.
Transplantation from Unrelated Donors
After the initial success with transplantation of marrow from matched unrelated volunteers, large databanks were established around the world, including the National Marrow Donor Program (NMDP) in the United States. Available through the world-wide registries and the NMDP are approximately 20 million volunteer donors who have been typed for HLA-A and -B antigens, and many of these are also typed for HLA-DR (www.bmdw.org). Certain racial and ethnic groups are underrepresented in the registry and therefore there is a lower probability that an HLA-matched donor will be found.
Outcomes have improved after HCT from HLA-matched unrelated donors because of improved supportive care and high-resolution HLA typing. Outcomes after HCT from an unrelated donor are now comparable to what is observed after HCT from an HLA-identical sibling. In a study of 2,223 adult AML patients, the overall survival after transplantation from 8/8 HLA-matched donors was similar to that observed after transplantation from an HLA-identical sibling (
RR = 1.03;
P = 0.62).
100 The risk of acute GVHD, however, was lower after transplantation from an HLA-identical sibling.
The relative importance of the various HLA loci has been defined for HCT from unrelated donors. In a study from CIBMTR of 3,857 transplants performed from 1988 to 2003, it was observed that high-resolution DNA matching for HLA-A, HLA-B, HLA-C, or HLA-DRB1 (8/8 match) was the minimal level of matching associated with the highest survival.
101 A single mismatch at HLA-A, HLA-B, HLA-C, or HLA-DRB1 (7/8 match) was associated with an increased mortality compared to an 8/8 match. A single mismatch at HLA-B and HLA-C appeared better tolerated than a mismatch at HLA-A and HLA-DRB1. A mismatch at HLA-DQ or HLA-DP loci did not have an effect on survival. Ninety-four percent of the transplants in this study were performed with marrow grafts. In a follow-up study, the association between HLA matching and outcomes was investigated for transplantation of peripheral blood stem cells from unrelated donors.
102 Survival was better with 8/8 HLA matching compared to 7/8 HLA matching. Single HLA-C antigen mismatches were associated with an increased risk of treatment-related mortality and grade III-IV acute GVHD. HLA-B antigen/allele mismatching was associated with an increased risk of grade III-IV acute GVHD with no effect on survival. No significant differences in outcomes were observed with HLA-C allele mismatches, HLA-A antigen/allele mismatches, or HLA-DRB1 mismatches compared to 8/8 HLA-matched pairs. The differences in the reported associations for HLA mismatches between marrow and peripheral blood stem cell grafts may result from differences in cell numbers in the graft as well as the graft composition.
Disease status is an important consideration in the search process. In patients with advanced malignancies, survival after transplantation from an HLA-matched or 1-antigen/allele HLA-mismatched unrelated donor was similar.
103 Therefore, when transplants cannot be delayed because of disease status, selection of a donor with the fewest HLA mismatches may be an alternative choice for patients without a completely HLA-matched donor.
Because of the possible higher risk of acute GVHD, studies of peripheral blood stem cell transplantation from unrelated donors started later than those done from HLA-identical donors. A recently completed randomized clinical trial (BMTCTN 0201) comparing marrow to peripheral blood stem cells observed that there was no difference in overall survival, disease-free survival, nonrelapse mortality, relapse, or acute GVHD outcomes at 2 years between the 2 arms. There was a significantly increased risk of chronic GVHD.
Transplantation with Umbilical Cord Blood Grafts
There are several advantages to the use of umbilical cord blood when compared to unrelated donor peripheral stem cell or marrow product harvested from adults. First, umbilical cord blood represents a potentially nonlimiting donor source for transplantation. At present, over 550,000 HLA typed cord bloods are banked and are conceivably available with several days notice (www. bmdw.org). Many of the banked specimens are from underrepresented ethnic and racial groups, thus expanding the potential donor pool for individuals poorly served by the unrelated donor marrow registries. The total nucleated cell dose required for successful engraftment is 10-fold less than that required for conventional peripheral blood or marrow transplant. The transmission
of Epstein-Barr virus (EBV) or cytomegalovirus (CMV) is negligible compared to conventional allogeneic transplantation. HLA allele mismatches (up to 2 to 3 loci) are permissible in cord blood transplantation. Notable disadvantages to cord blood transplantation are the potential for prolonged pancytopenia and lower rates of overall engraftment.
The first successful cord blood cell transplant in a pediatric patient was done in 1988.
69 Two studies were then successfully conducted of cord blood transplantation from HLA-matched or HLA-haploidentical allogeneic siblings (44 patients) and partially HLA-mismatched unrelated donors (25 patients).
104,
105 In the first report of a large experience in 562 recipients (including 18% adults) after transplantation with umbilical cord blood grafts from unrelated donors, the probability of engraftment at 42 days and grade III-IV GVHD, was 81% and 23%, respectively.
106 It was concluded from this study that umbilical cord blood grafts regularly engraft and cause a low rate of GVHD relative to the number of HLA mismatches, and produced survival rates comparable to those with transplantation of marrow from unrelated donors. This experience was confirmed in a retrospective analysis of 541 children with leukemia transplanted with stem cell grafts from unrelated donors of which 99 were from umbilical cord blood.
107 Recipients of cord blood had an increased number of HLA mismatches but a lower risk of both acute and chronic GVHD compared to recipients of unmanipulated marrow from unrelated donors. The day 100 mortality was higher, however, in the cord blood group, possibly because of the significantly delayed recovery of hematopoiesis and immunity after transplantation. These results were later confirmed by another group.
108 It was concluded that the use of umbilical cord blood was an option for children with acute leukemia lacking an acceptably matched unrelated marrow donor. In children who had received umbilical cord blood or bone marrow grafts from HLA-identical siblings, the umbilical cord blood group had a lower risk of acute and chronic GVHD (relative risk 0.41 [
P = 0.001] and 0.35 [
P = 0.02]), respectively.
109 Survival was similar in both groups. The progenitor cell and CD34-positive cell content of the umbilical cord blood graft predicted the rate of neutrophil recovery after transplantation.
110The reported low incidence of severe GVHD after transplant of umbilical cord blood cells from unrelated donors (relative to the degree of HLA disparity) may be related to the decreased immunocompetence of the fetal blood cells compared to adult cells.
111 Two years after transplantation, T cell receptor rearrangement excision circles (TRECs), a measure of recent thymic output, were greater in recipients of umbilical cord blood than in recipients of marrow grafts, suggesting complete immune recovery despite the low number of cells infused.
112Initially, recipients of umbilical cord blood cell transplants had been mostly children. Even though, at first, there was a concern about the low cell count for the larger body size, outcomes in adults were comparable to those reported in children.
113 Prospective trials comparing cord blood transplantation to unrelated peripheral blood/marrow transplants are not available; however, two large registry or retrospective studies summarizing consortia experience for acute or chronic leukemias have been published.
114,
115 Slower engraftment kinetics, reduced engraftment rates, and decreased acute and chronic GVHD rates and severity were observed in the cord blood transplantation groups. Rates of relapse reported for the two studies were similar between the 2 groups. Transplant-related complications, including delayed recovery of blood counts after cord blood transplantation, may be reduced in the adult population with reduced intensity conditioning.
116Transplantation of multiple cord blood units was investigated to determine if recovery of neutrophil and platelet counts after transplantation could be improved.
117 After the infusion of two umbilical cord blood grafts into adult patients, the median day to neutrophil recovery was 24 (range 12 to 28) in 23 adult patients compared to 27 (range 13 to 59) in another study of 68 adult patients transplanted with a single cord blood unit.
113 This observation of improved recovery times was not confirmed in a retrospective study at a single institution.
118 After transplantation with 2 cord blood units, only one unit eventually dominates and engrafts long-term.
119 Higher CD3
+ cell dose and percentage of CD34
+ viability were associated with unit dominance. Higher dominant unit total nucleated cells, CD34
+ cells, and colony-forming unit doses were associated with higher sustained engraftment and faster neutrophil recovery. Mechanistically, the dominant cord mounts an allogeneic immune response mediated by CD8
+ T cells against the nondominant unit.
120 Outcomes after double cord blood transplantation were compared with outcomes after transplantation from HLA-matched related donors, HLA-matched unrelated donors, and 1-antigen HLA-mismatched unrelated donors.
121 Leukemia-free survival at 5 years was similar for all donor types. The risk of relapse was decreased in the double cord blood transplantation group compared to the other donor types, but the risk of nonrelapse mortality was increased. The lower risk of relapse after transplantation from a double cord blood unit compared to a single cord blood unit had been previously seen in another retrospective study, although a significantly increased risk of acute GVHD was also observed.
118 Sharing of one or more HLA-A, B, or DRB1 loci between the inherited paternal MHC alleles (IPA) of the donor and the recipient after unrelated cord blood transplantation leads to superior leukemia-free survival with no increase in acute GVHD, a result attributed to maternally derived anti-IPA elements persisting in the cord blood.
122Umbilical cord blood grafts should be considered for both pediatric and adult patients lacking a suitably matched unrelated donor or unable to wait for an unrelated search to be completed.