INTRODUCTION
Monocytes, macrophages, and dendritic cells (DCs) constitute a group of myeloid cells which share common hematopoietic origins and express related functions in host homeostasis and innate and acquired immunity. They develop in hematopoietic organs, enter the circulation, and are widely distributed throughout almost all tissues. They express a variable capacity for migration, phagocytosis, antigen presentation, and secretion.
Study of this family of cells, initially known as the reticuloen-dothelial system (RES), and later as the mononuclear phagocyte system (MPS), is undergoing rapid change, with recent emphasis on cellular heterogeneity and differentiation, compounded by complex modulation of their phenotype within tissues. As a result of their specialized functions, the fields of macrophage and DC immunobiology have diverged to a great extent. Our goal in this chapter is to re-integrate these divisions, to emphasize their related properties and variations on a common theme. While much remains unknown concerning their life history and properties in situ, there is a considerable body of detail now available from studies in cell culture and in experimental animals, especially in the mouse, which may not necessarily reflect their behavior in humans. In this chapter, we summarize their cellular and molecular properties and emphasize studies in humans, drawing attention where possible to clinically relevant functions and pathogenic mechanisms. While primarily aimed at hematologists, this chapter highlights the major populations present in the
tissues, both normally and in a range of inflammatory, infectious, metabolic, and neoplastic diseases.
In the following section, we summarize general and specialized features of monocytes, macrophages, and DCs, collectively termed mononuclear phagocytes (MPs).
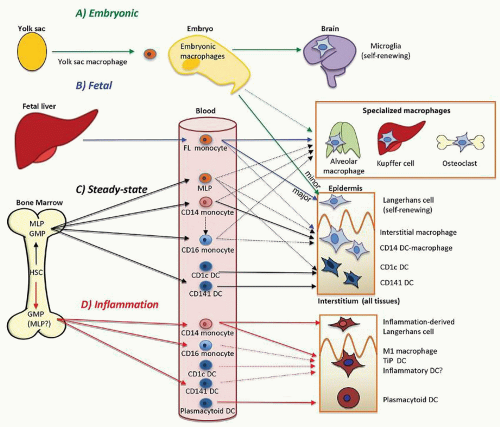
Figure 10.1 illustrates a compre-hensive overview of human MP differentiation and distribution, to be discussed in detail in subsequent sections.
FUNCTIONAL PROPERTIES
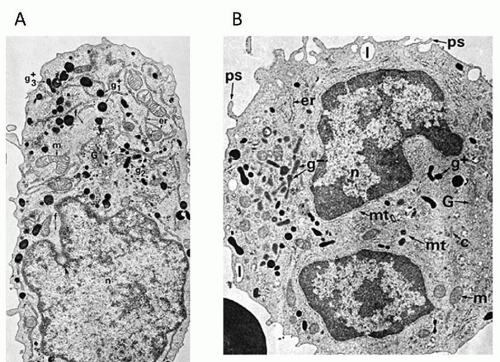
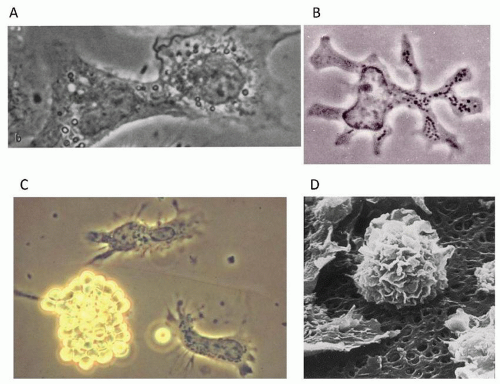
Monocytes are rounded cells, 10 to 15 µm in size, with oval, kidney-shaped, or indented nuclei, a rim of heterochromatin, and mostly euchromatic nucleoplasm. Their cytoplasm is relatively abundant, compared with nonactivated lymphocytes, containing myeloperoxidase+ and rudimentary lysozyme+ granules, nonspecific esterases, and lysosomes. Scanning electron microscopy reveals extensive surface folds. They adhere readily to native and artificial substrates by a range of adhesion receptors, flattening out and spreading in characteristic fried egg or stellate shape with fine plasma membrane processes. They respond slowly to chemotactic gradients in vitro, compared with neutrophils.
Macrophages are larger, rounded (e.g., in alveolar space, peritoneal cavity), or stellate, with two or more processes, sticky and sluggish though motile, and with extensive, dynamic plasma membrane processes and filopodia. Podosomes have been observed. The cytoplasm of cultured cells has a well-developed centrosome, with an organized cytoskeleton, and is rich in synthetic organelles and endocytic vesicles, often containing debris and residues of phagocytosis in abundant lysosomes. They lose their peroxidase granules as monocytes mature in culture. Macrophages love lining up along surface irregularities on culture substrata and usually keep their distance from one another in culture; inflammatory stimuli cause aggregation. Occasional binucleate cells arise by failure of cytokinesis; macrophage fusion can be observed “spontaneously,” especially on selected substrates, or after exposure to serum lipids or cytokines such as interleukin (IL)-4.
DCs in the blood are smaller than monocytes and may be found in the “lymphoid” gates on flow cytometry analyses. In the tissues, they are smaller than macrophages and less extensively arborized with multiple short processes. During migration through the afferent lymph they mature into “veiled cells” with extensive macropinocytic processes. In lymph nodes they are highly motile and transit rapidly between T cells until a cognate antigen interaction is detected by the T cell. In vitro they are lightly or nonadherent compared with monocytes or macrophages, a property that was exploited in their original isolation from mouse spleen.
Chemotaxis, Migration, and Adhesion
See
Figures 10.5 and
10.6A. Selected antigen markers and receptors are listed in
Tables 10.2 and
10.3.
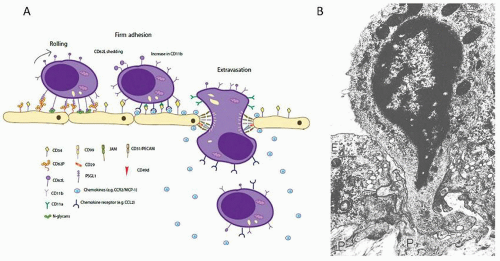
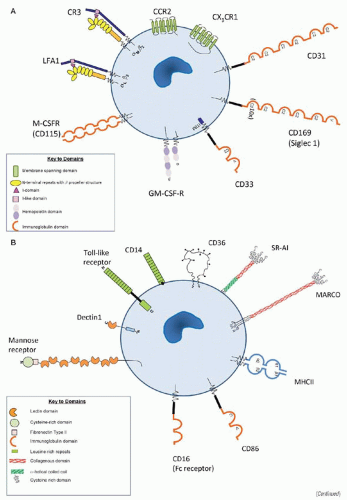
11,12,13,14,15,16,17,18,19,20,21 and 22,23,24,25Chemotaxis of MPs to inflammatory sites is stimulated by factors such as complement component C5a, N-formylated oligopeptides, fragments of fibronectin, elastin, and collagen; and by secreted proteins called chemokines. Stimulation by chemotactic factors results in increased integrin affinity and therefore binding to the endothelium at the same time as formation of lamellipodia and actin polymerization, resulting in cell movement. Chemokines are divided into subclasses on the basis of the spacing of the N-terminal cysteine residues. CCL#, CXCL#, CL#, and CX3CL# refer to four families of chemokine ligands, in which # denotes the identifying number, C denotes a cysteine, and X denotes a noncysteine amino acid. CC chemokines are responsible for attracting monocytes and lymphocytes, directing traffic of leukocytes under steady state conditions; whereas inflammatory chemokines are expressed by circulating leukocytes and other cells only upon activation. In the mouse two subsets, classical (Ly6C+ analogous to human CD14++CD16−) and nonclassical monocytes (Ly6Clo, human CD14dimCD16+) express different chemokine receptors, especially CCR1, CCR2, and CX3CR1 (fractalkine receptor). Under conditions of inflammation, monocytes are recruited to tissues by CCL2 (MCP-1), CCL3 (MIP1a), and CCL5 (RANTES) acting through CCR1, CCR2, and CCR5. These inflammatory monocytes are able to differentiate into macrophages or DCs that appear to play important roles in the initiation of immune responses. The signals that drive steady state recruitment have not been solved but are independent of CCR1, CCR2, and CCR5.
Monocytes and macrophages adhere to other cell types, including lymphocytes and vascular endothelial cells, and to extracellular matrix components, e.g., fibronectin and laminin, using specific cell surface adhesion receptors. Inflammatory macrophages and DCs originate from the migration and differentiation of circulating monocytes into virtually all tissues, contributing to
the pathophysiology of many human diseases including atherogenesis and other inflammatory diseases. The origin of steady state tissue macrophages and DCs is much less certain, as discussed below. It is likely that blood monocytes and blood DCs contribute, but circulating hematopoietic progenitor cells may also give rise to tissue leukocytes.
Three families of cell surface glycoproteins mediate most cell adhesion: integrins, immunoglobulin-related molecules, and selectins. Integrins are large heterodimeric glycoproteins classified in subfamilies according to the common beta subunit; beta 1 (CD49/cd29) or VLA, beta 2 (CD11/CD18), beta 3 (cytoadhesion), and beta 7 are the most relevant. Integrins recognize Ig-related molecules such as intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and vascular cell adhesion molecule (VCAM). Selectins (L-selectin, E-selectin, and P-selectin) recognize oligosaccharides, e.g., Sialyl Lex. CD44 and CD36 are structurally unrelated but important adhesion molecules which bind to hyaluronic acid and thrombospondin. Macrophage entry into tissues is a multistep process involving both adhesion and transendothelial migration. The process involves monocyte tethering and rolling on the surface of activated endothelial cells mediated mainly by activated selectins; firm adhesion is mediated by VCAM-1 and ICAM-1 on the endothelium, binding to β1 and β2 integrins expressed on leukocytes. Subsequently, monocytes transmigrate the endothelium.
The dynamic complexity of adhesion receptor utilization in chronic inflammatory processes such as atherosclerosis is exemplified by the following studies. Analysis of integrin, immunoglobulin-related, and selectin expression on blood monocytes, in vitro differentiated macrophages, and alveolar macrophages reveals monocyte expression of β1 (CD29), α4, α5, α6, β2 (CD18), CD11a, CD11b, and CD11c subunits, but not αV (CD51). Some expression of CD41b (IIb) and CD61 (β3) has been detected. The Ig-related molecules CD54 (ICAM-1), ICAM-2, and CD58 (LFA-3) are expressed, as well as L-selectin and the carbohydrate ligands Lex (CD15) and sialyl Lex. CD44 and CD36 are strongly positive. Alveolar macrophages exhibit lower expression of α4, α6, β2, CD11a, CD11b, L-selectin, Lex, and sialyl Lex. ICAM-2 and CD36 are absent, whereas expression of α3, but not of CD11c, is higher. Similar results were obtained with in vitro differentiated macrophages. The profile of adhesion receptors expressed as monocytes differentiate into macrophages varies according to tissue location and the disease state. For example, endothelium overlying human atherosclerotic lesions and fatty streaks expresses high levels of E-and P-selectins, ICAM-1, and VCAM-1. Selectin deficiency reduces atherosclerosis, and genetic mutation of ICAM-1 or VCAM-1 reduces atherosclerosis in mice. Oxidized LDL leads to endothelial dysfunction, leading to expression of adhesion molecules and recruitment of monocytes into the subendothelial space. Ox-LDL is taken up by macrophages via scavenger receptors such as SR-A1, SR-A2, and LOX-1, which are themselves implicated in local macrophage adhesion. CD36, a multifunctional membrane receptor present on MPs and other cells, functions as a scavenger receptor for oxidized phospholipids. On macrophages, CD36 interaction with oxidized LDL triggers a pro-inflammatory and pro-atherogenic response involving activation of src-family kinases, MAP kinases, and Vav family guanine nucleotide exchange factors and results in ligand internalization, foam cell formation, and inhibition of migration.
Macrophage adhesion molecules also have an important role in homotypic cell fusion, forming osteoclasts and multinucleated giant cells associated with chronic inflammation. Progress has recently been made in identifying molecules involved in macrophage fusion. Signaling processes mediated by DAP12 and STAT6 induce a fusion-competent status. Chemotaxis through CCL2, cell-cell adhesion mediated by E-cadherin, exposure of phosphatidylserine, lipid recognition by CD36, purinergic receptors, and cytoskeletal rearrangement dependent on RAC1 are prerequisites for successful macrophage fusion.
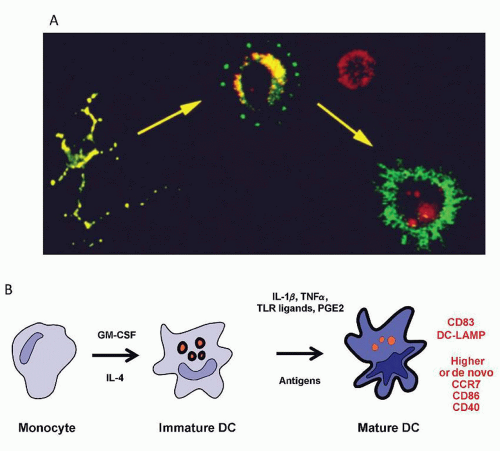
The regulation of DC function is intimately linked to migration. At sites of antigen contact, myeloid or “classical” DCs are found in immature forms with high expression of pattern recognition receptors (PRRs) and endocytic activity, but low surface expression of MHC and costimulatory molecules. Migration and maturation are believed to occur in the steady state, but are massively increased by local inflammatory stimuli mediated by TLR agonists, cytokines, and other danger signals. DC migration from tissue sites to afferent lymphatics is integrin-independent and driven by chemokine gradients of CCL19 and CCL21 acting through CCR7. Recent studies show that these chemokines decorate the endothelium of afferent lymphatics. The passive drainage of fluid through afferent lymphatics may also play a part in the migration of DCs towards lymph nodes. Within the node, similar chemokine gradients position DCs in the T cell areas, where they upregulate antigen-bearing MHC molecules and costimulatory antigens. Antigen uptake declines and morphological changes ensue, including the extension of dendrites. Myeloid and plasmacytoid DCs also enter the lymph nodes directly from the bloodstream, where they undergo a parallel process of maturation. In the later stages of immune activation, monocyte-derived DCs also reach the lymph nodes by direct recruitment from the blood or via inflamed tissues.
Recognition: Plasma Membrane Antigen Markers, Sensors, and Regulators
See
Figures 10.6,
10.7,
10.8,
10.9,
10.10,
10.11 and
10.12 and
Tables 10.1,
10.2 and
10.3.
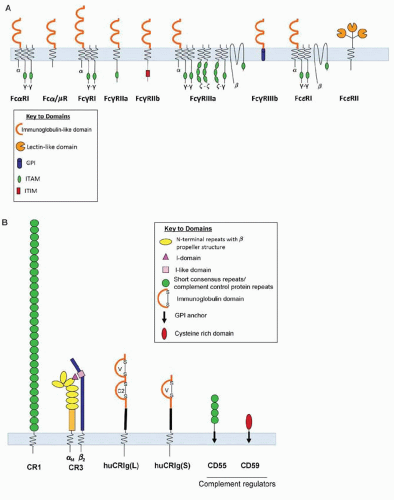
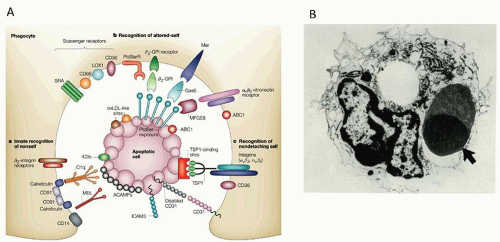
26,27,28,29,30,31 and 32,33,34,35,36,37Monocytes and macrophages express a range of opsonic and nonopsonic plasma membrane and endosomal receptors to recognize foreign and modified self-ligands. These include activatory (ITAM cytoplasmic motif) and inhibitory (ITIM motif) Fc receptors, receptors for complement-derived ligands, TLRs, lectins, and scavenger receptors, as well as receptors for other humoral ligands, for example, collectins and pentraxins. Monocytes also express CD14, a GPI-linked receptor for LPS, which is downregulated upon cell maturation in culture. Resting monocytes do not express class A scavenger receptor I/II, which is upregulated on macrophages during maturation, but do express MARCO,
a related SR, and CD36, another promiscuous adhesion molecule also present on platelets and endothelial cells. The lectins include Dectin-1, the receptor for fungal
β-glucan, expressed by monocytes, macrophages, neutrophils, and DCs; the mannose receptor, a multilectin with distinct carbohydrate recognition domains for GlcNac, mannosyl, and fucosyl ligands on microbes and selected host molecules; and a cysteine-rich domain which recognizes endogenous sulfated sugars. Other mannose binding lectins include DC-SIGN and Dectin-2; galectin is a receptor for galactosyl recognition. Additional plasma membrane receptors include CD163, an SR-like molecule that recognizes haptoglobin – hemoglobin complexes, and which is induced by glucocorticoids. Additional SR, not shown, are present on endothelial cells, which also express SR-A. Apart from the scavenger receptors, several other receptors can recognize apoptotic cells, e.g., the Tyro3, Axl, and Mer (TAM) receptors, which recognize Gas 6 and Protein S ligands. Several TIM molecules and CD300A bind phosphatidyl serine, another ligand for apoptotic cell recognition.
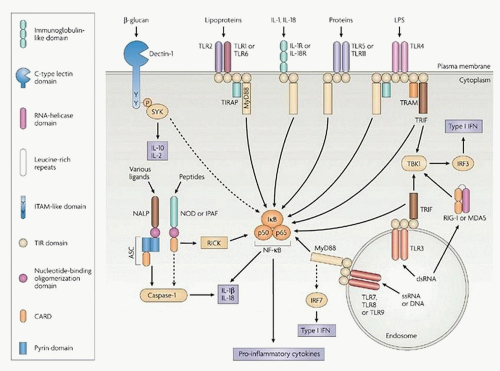
In common with monocytes and macrophages, DCs are equipped with an array of PRRs, which can detect evolutionary-conserved pathogen-associated molecular patterns (PAMPs), including proteins, lipids, carbohydrates, and nucleic acids. The PRRs encompass families of membrane-bound TLRs and C-type lectin receptors (CLRs), such as Langerin, MMR, DC-205, DC-LAMP, DC-SIGN, Dectin-1, and DCIR. The PRRs also include cytosolic NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and helicase nucleic acid receptors, as discussed further below. Human DCs are equipped with multiple CD1 antigens for the recognition of bacterial lipid antigens (notably CD1a and CD1c) and a range of receptors for complement (CD11b and CD11c) and coagulation proteins (CD141: thrombomodulin).
The SIGLEC family of leukocyte plasma membrane molecules interacts with sialylated glycoconjugates, and members are variably expressed on myeloid cells of different species, including macrophages and plasmacytoid DCs. Siglec-1 (sialoadhesin, CD169) has been implicated in binding of hematopoietic cells, including lymphocytes.
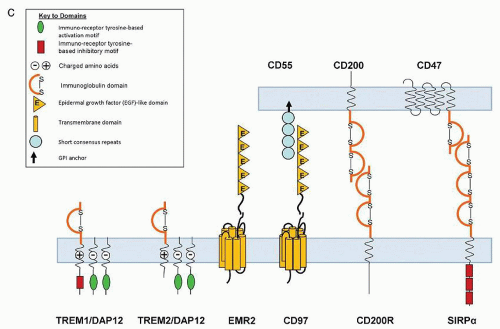
The murine macrophage plasma membrane antigen marker F4/80 is part of a small family of seven transmembrane adhesion GPCR which includes CD97 and EMR2, more broadly expressed on myeloid cells. EMR2 is a useful immunocytochemical marker for human tissue macrophages, as well as neutrophils, in which it potentiates a range of cellular functions. The TREM family also modulates myeloid cell responses; TREM-2, for example, associates with DAP-12 as one of several signaling partners. Counterreceptors include the negative receptor pair CD200/CD200R.
Genetic polymorphisms have been described for a range of the above surface molecules; for example, TLR mediating pro-inflammatory signaling and Fc receptors, which mediate important functions such as phagocytosis, antibody-dependent cytotoxicity, and immunomodulation.
Cytosolic Recognition and Inflammasome Activation
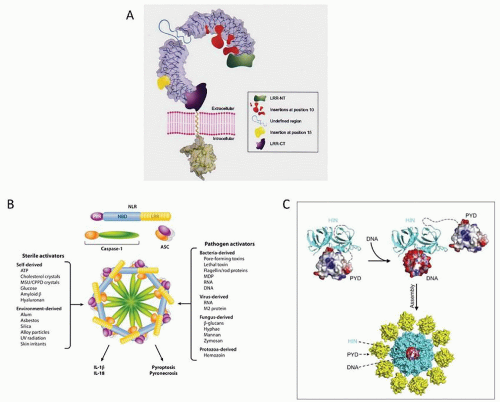
This topic has benefited enormously from the study of auto-inflammatory syndromes associated with rare genetic
disorders, and their dramatic amelioration by IL-1 antagonists. Inflammasomes are protein complex platforms which are required for the activation of inflammatory caspases and the maturation of their pro-inflammatory cytokines including IL-1
β and IL-18. They are constructed around several proteins, including NLRP3, NLRC4, AIM2, and NLRP6, and recognize inflammatory signals arising from receptors for PAMPs, damage-associated molecules (DAMPs), and sterile particulates, such as uric acid crystals. Recognition of immune signals by one of several families of receptors results in direct activation of caspase-1 and caspase-5, secretion of potent pro-inflammatory cytokines, and a form of cell death called pyroptosis. PRRs of innate immune cells can be classified into phagocytic and sensor PRRs and, in addition to the plasma membrane receptors described above, include intracellular TLRs, retinoic acid-inducible gene I-like receptors (RLRs), and nucleotide-binding oligomerization domain-like receptors (NLRs). Inflammasome-mediated processes are important during microbial infections and also in regulating both metabolic processes and mucosal immune responses. For example, the NLRP3 inflammasome has
been demonstrated to be involved in antibacterial, viral, fungal, and parasitic immune responses. On infection with influenza A, endosomal TLR7 recognizes viral RNA and induces transcription of the NLRP3 inflammasome components. Selected bacteria have been shown to allow the cytoplasmic entry of flagellin, the NLRC4 ligand, leading to activation of the NLRC4 pathway. Some inflammasome activators are exogenous particles, e.g., silica and asbestos, whose uptake by pulmonary macrophages activates NLRP3 inflammasome-dependent caspase activation, cytokine, and cellular reactive oxygen species release, contributing to silicosis or asbestosis. Inflammasomes have also been implicated in metabolic pathologies with activation of caspase-1 by NLRP3 in adipose tissue, resulting in inhibition of insulin signaling, expression of TNF-
α, and induction of CD4 T helper cells. However, a protective role for activated inflammasomes in age-related macular degeneration has also been proposed, since lack of NLRP3 or IL-18 exacerbates choroidal neovascularization.
Intracellular detection of nucleic acids by DCs is very sophisticated. TLR3, TLR7, and TLR9 cooperate with a large family of helicases, RLRs, and DNA sensors in the recognition of single- and double-stranded nucleic acids. Ligation of these receptors leads to rapid phenotypic maturation, activation of antigen-presenting capacity, and cytokine release; plasmacytoid DCs, for example, release abundant type 1 interferon in response to viral infection.
Endocytosis and Phagocytosis
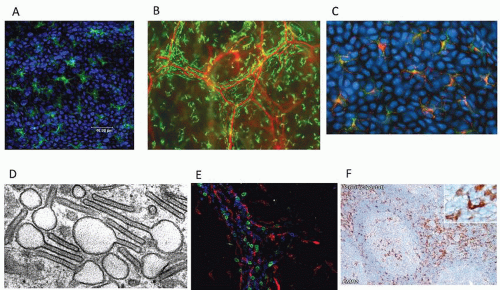
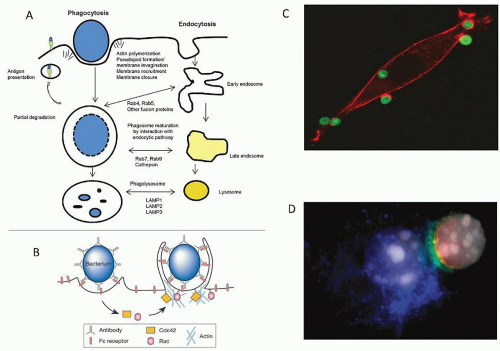
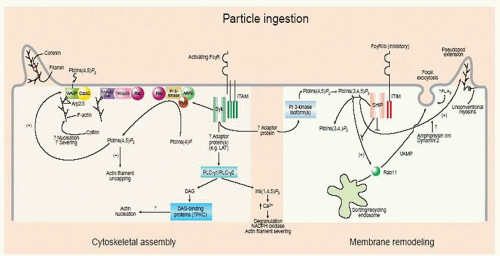
Monocytes and macrophages are able to internalize and ingest soluble and particulate ligands, including apoptotic cells and micro-organisms, with variable efficiency. Surface molecules which mediate receptor-mediated endocytosis, apart from those listed above, include transferrin (CD71) and folate receptors. Macrophages are able to internalize the equivalent of their surface in 20 minutes; and a series of fusions and fissions, assisted by small GTP-ases, result in membrane retrieval, receptor recycling to the cell surface, or cargo delivery from endosomes to lysosomes for digestion. Phagosome formation and fusion result in formation of phagolysosomes. Fc receptor-mediated uptake depends on circumferential engagement of the target ligands by pseudopodia, a zipper-like process, and ingestion depends on the actin cytoskeleton. Complement-mediated uptake proceeds by a distinct “sinking” mechanism. Underwood and colleagues have described formation of a phagocytic synapse in the uptake of yeast particles
by Dectin-1, which has a hemi-ITAM in its cytoplasmic domain, by a syk- and CARD9-dependent pathway. Uptake is associated with acidification, to promote digestion of all macromolecules.
Endocytosis by DCs follows special pathways that preserve antigens for presentation on MHC molecules. Most DCs are competent to present externally acquired antigen to maturing MHC class II dimers in the late endosomes. A complex pathway involving invariant chain and HLA-DM maintains MHC class II in an open configuration for peptide binding. A subset of DCs is competent at cross-presenting externally acquired antigen to Class I MHC. These
DCs are characterized by high expression of CD141 in humans and CD8 in mice. They also possess a unique chemokine receptor XCR1 and lectin CLEC9A (DNGR-1) with actin as ligand; and they express a high level of TLR3. Specialized biochemical machinery allows these cells to transfer exogenous antigen to the endogenously loaded MHC class I presentation pathway.
Autophagy is an analogous process by which macrophages and DCs surround damaged cytoplasmic organelles with membrane for delivery to lysosomes, especially after microbial infection. Several components involved in this process have been identified and some have been implicated in genetic predisposition to inflammatory bowel disease. This is also thought to be a key process in antigen presentation of cytosolic antigens to MHC class I.
Signaling and Gene Expression
A range of signaling pathways have been characterized in myeloid cells following on receptor ligation and collaboration, which differ depending on the cell type involved. These include sequential phosphorylation/dephosphorylation by kinases and phosphatases, the assembly and disassembly of protein complexes, and redistribution of molecules including phosphoinositides and lipid metabolites from cytoplasm to the plasma membrane as well as to the nucleus. The adaptor proteins for TLR-signaling include MyD-88, TRIF, and TIRAP/MAL. Signaling pathways that are particularly well characterized include MAP kinase activation, classical and alternative NFkB activation, and GPCR activation, e.g., by leukotrienes. Actin assembly and disassembly play a prominent role in cell migration and phagocytosis, as well as secretion. Gene expression depends on dynamic changes in chromatin conformation, binding and release of transcription complexes, and epigenetic modifications of DNA as well as histones by methylation and acetylation. Recent studies have begun to compare transcription by monocytes, macrophages, and DCs in considerable detail, including microRNA analysis. Translational modifications include glycosylation, prenylation and other lipid modifications, and ubiquitination, which determine protein turnover and export, as well as biosynthetic quality control. Current high throughput sequencing and microarray and proteomic analysis is set to transform our knowledge of cell functions and differences as well as of similarities among monocytes, macrophages, and DCs. We include a few recent references of useful resources in a fast-moving field.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access