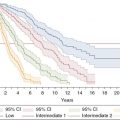
Clinical and pathogenetic changes occurring during myeloproliferative disorder progression. MPN, myeloproliferative neoplasm; MPN-BP, MPN blast phase; ET, essential thrombocythemia; PMF, primary myelofibrosis; PV, polcythemia vera; MF, myelofibrosis; LDH, lactate dehydrogenase.
The incidence rate of MPN-BP is lowest in ET, ranging from ~1% to 4%; it is around 4–8.7% in PV and ~15–22% (up to ~30% in some studies) for primary myelofibrosis.5,6 The time to onset of MPN-BP varies but is long, measured in many years to decades.
Several studies examined risk factors to predict time to onset of MPN-BP based on clinical and chromosomal information. The individual studies assessed different metrics over time and used different scoring systems, hence there are some variations in the clinical parameters used to predict a higher risk of progression into MPN-BP. Commonly associated prognostic factors for an increased rate of transformation include leukocytosis (>30 × 109/L or >25 × 199/L in the Dynamic International Prognostic Scoring System-Plus (DIPSS-Plus)), thrombocytopenia (<100–150 × 109/L), and anemia (<10 g/L) (with respect to time to onset of anemia),7 as well as unfavorable karyotype (either defined within prognostic risk scores such as the Lille Score or the newer DIPPS or DIPSS-Plus for individual chromosomal aberrations) or defined as complex karyotype with >3 aberrations, similar to AML.2,4,6,8,9 In most studies, leukocytosis, increased blood blasts (>1–3%), progressive anemia, and thrombocytopenia, as well as monosomal or complex karyotype, seem to play particularly important roles.2,4,6,8,9 Finding a monosomal/complex karyotype or other unfavorable cytogentic chromosome aberrations that were not present previously indicates clonal progression. Practically, if we see patients whose leukocytosis and/or peripheral blood blasts steadily rise (i.e., from 2% to 6% and 10% over a 6–12-month period), with progressive cytopenias, this may necessitate a marrow biopsy and closer observation and initiate the process of possibly proceeding to an allo-SCT. Comparable to myelodysplastic syndrome (refractory anemia with excess blasts, RAEB-2) the higher the percentage of blasts (i.e., >10% in marrow in some studies), the higher the risk of progression. There is a higher frequency of a FAB morphological M6 and M7 subtype in MPN-BP leukemias compared to de novo AML.4
Hydroxyurea, if given alone or if not used concurrently or sequentially with cytoreductive therapy (mainly alkylators or radioactive phosphorus, 32P) does not appear to increase the risk of LT. However, 32P and alkylator treatment during the disease course were associated with 4.6- and 3.9-fold increased risk of LT.10 Thus, in younger patients one needs to be careful of the sequence and the agents used during the entire treatment time.
There are attempts to characterize which patients may be at higher risk for LT if hydroxyurea or other cytoreductive drugs are used. For example, in a recent study of four genes involved in pathways repairing DNA damage and providing protection against genotoxic DNA damage (in this study, nucleotide excision repair and base excision repair pathways), a specific Gln/Gln genotype in the XPD (ERCC2) gene at codon 751 showed the strongest association with LT (odds ratio, 4.9; 95% confidence interval, 2.0–12) but was also associated with development of non-myeloid malignancies.11 Data such as these provide evidence about the contribution of inherited genetic variations to the pathogenesis and clinical/treatment course of MPNs and other malignancies, with the hope one day of testing for specific higher-risk genetic predisposition in patients who are at risk. As of today, prudent use of sequential or concurrent alkylators with hydroxyurea should be exercised.
Recognizing disease acceleration of myelofibrosis heading towards LT is important, as the median survival of patients with MPN-BP is only approximately 3–6 months4,5 in earlier studies. It may be slightly higher in recent papers (Table 21.1), possibly due to an effect of aggressive management and close observation of patients at risk, and proceeding quickly to allo-SCT.12 Thus, early recognition of disease acceleration and initiating allo-SCT evaluation are crucial as allo-SCT is the best (and currently only) chance for a potential cure. Ideally allo-SCT should be performed prior to the onset of LT in the accelerated phase of myelofibrosis (see below).
| Therapy | Composition | Median survival in months (range) |
|---|---|---|
| Supportive care | 2.0 (0–20.1) | |
| Non-induction chemotherapy Hypomethylating agents | 2.9 (0.4–22.5) 8 (NR) 9 (5–45+) | |
| Induction chemotherapy | 3.9–9.4 (1.6–57) | |
| Allogeneic stem cell transplantation |
| 3.9–10.5 (3–47) |
Molecular mechanisms of leukemic transformation and MPN-BP
The exact etiology and molecular processes leading to LT are unknown. However, several of the commonly found mutations in myeloid malignancies (MPNs, AML, myelodysplastic syndrome) seem to occur at higher frequency in MPN-BP vs. the chronic state of the respective MPN, indicating a possible contribution to LT. This chapter cannot adequately reflect on the complex molecular aspects of MPN-BP and we refer the reader to other sources.
Does JAK2 play a role?
JAK2 mutations are the most common mutations. Some papers report an increase in the JAK2 allele burden from heterozygous to homozygous upon progression within the myelofibrosis stage. However, JAK2 is not necessarily involved in transformation and there seem to be alternate JAK2-independent routes to LT, which may even be more common.13 In another study, all JAK2 V617F patients remained JAK2-positive and all JAK2 V617F-negative cases remained negative at LT, reinforcing the notion that other molecular event(s) may play a role in the clonal heterogeneity of MPNs. Thus, in addition to the well-described clonal evolution at the cytogenetic level, additional genetic and molecular changes apart from JAK2 mutation and signaling promote LT. A detailed description of the molecular and genetic events or genes involved is beyond the scope of this chapter, especially as there is still controversy about the contribution of each of these events in the pathogenesis of LT. Below we will highlight frequencies and occurrences of mutations and their differences between the chronic state of MPNs and MPN-BP.
Other mutations in MPN-BP
Mutations that have been found at a higher freqeuncy in MPN-BP versus the initial MPN (PV, ET, and primary myelofibrosis) are 21% vs. 1–4% for IDH1 and IDH2 mutations; DNMT3A, 14% vs. 3–7%; LNK mutations are around 10% in MPN-BP vs. rare occurrences in PV/ET/primary myelofibrosis; ASXL1 18% vs. 3–13%, and SRSF2 has been reported at 18.9% in MPN-BP. TET2 mutations appear to be equally common in MPN-BP (~17%), PV (16%), and primary myelofibrosis (17%).14 It should be emphasized that different studies conducted in different patient populations from different groups have slightly varying frequencies of mutations; for example, in a recent study of 54 MPN-BP patients, TET2 mutation frequency was 28.3% and that for DNMT3A only ~2%.15
Karyotype
On LT, there is an increase in complex karyotype, indicating the acquisition of additional genetic damage and lesions. For example, in our observation a complex karyotype with three or more abnormalities was found in around 18.7% and baseline and 53.6% at leukemic transformation,4 confirmed by other studies to be in a similar range.16 Acquisition of deletion/monosomy of chromosome 5, 7, and trisomy 8 were common. This is similar to other acute leukemias, where a more complex karyotype indicates a worse disease biology. In addition to cytogenetic abnormalities, an increasing number of mutations have been identified that have a role in the initial formation of MPN/myelofibrosis,14 as well as, later on, transformation into acute leukemia. Several specific cytogenetic alterations have been observed at loci that harbor genes known to be involved in leukemogenesis; for example, deletion in 7p corresponding to the transcription factor IKZF.17 Mutations/loss of the tumor suppressor gene p53 are higher as well.
Chemotherapy and allogeneic stem cell transplantation
Treatment of MPN-BP is challenging and there is no standard of care. Many of the treatment concepts are derived from AML. Several groups, including our group, have reported on the outcome of myelofibrosis at LT.4,12,18,19 Patients eligible for induction therapy should undergo chemotherapy. However, achieving complete remission in MPN-BP has not, or only modestly, improved overall survival, although a complete response is still a prerequisite to proceed to allo-SCT. Therefore, we generally treat patients with induction chemotherapy if there is a plan to proceed to allo-SCT. Since there is usually a time interval at the stage of myelofibrosis, donor and transplant issues can be planned and available donor determined.
The choice of induction chemotherapy varies and the same regimens as in AML are used. The CR and CR with incomplete count recovery (CRi) rates are comparable to poor-risk de novo or secondary AML and are broadly in the range of 40–50% for the general MPN-BP population,4,12,18,19 although some studies show up to 71% CR/CRi with standard AraC plus anthracycline.18 The response kinetics and time-to-count recovery are also delayed given the significant bone marrow fibrosis in these patients. Overall survival ranged from 2.7 to 6.6 months for the general population. Overall survival in patients receiving chemotherapy and a CR was in the range of 3.9–9.4 months, again showing a broad range, but with only minimal improvement of survival after chemotherapy alone.4,12,18,19 Long-term survival is possible in selected patients, with some patients being alive at follow-up of up to 47 months posttransplant (Table 21.1). A few studies analyzed the outcome after combined induction chemotherapy followed by hematopoietic allo-SCT for eligible patients, showing median survival rates in the order of 10.5 with a range of up to to 47 months, clearly better than with chemotherapy or lower-intensity treatments alone, but still rather poor overall. In our recent series of MPN-BP patients, approximately 40–50% achieved 20 months or longer survival rates, and an important factor for long-term survival is achievement of a CR,19 if followed by allo-SCT.
A large recent retrospective study confirmed the validity of a systematic “aggressive” treatment approach consisting of close momitoring of patients during their myelofibrosis stage and induction chemotherapy followed by allo-SCT for eligible patients.12 Of 75 MPN-BP patients, 38 were eligible to receive induction chemotherapy; 18 achieved a CR/CRi and 11 a second chronic phase of MPN; 17 of the 38 chemotherapy patients proceeded to allo-SCT. Patients treated with this curative-intent approach (e.g., chemotherapy and/or planned to be followed by allo-SCT) (similar to an intention-to-treat analysis) had a median survival of 9.4 months and a 2-year overall survival of 26%, much better than the 2.3 months and 3% 2-year overall survival in the group that received non-curative lower-intensity or supportive-care approaches. Outcome was even better for the patients who completed allo-SCT, with a 2-year overall survival of 47% (median overall survival of 47 months) vs. 17% (median overall survival of 9.4 months) for those who achieved CR/CRi in the second chronic phase of MPN after induction chemotherapy, but who did not go on to allo-SCT. Importantly, patients undergoing induction chemotherapy but not proceeding to allo-SCT had a similar median overall survival compared to the low-intensity treatment group (9.4 vs. 6.6 months, respectively). Similar results were found by us and other groups.
In conclusion, MPN-BP should be treated with induction chemotherapy followed by allo-SCT for eligible patients. Which induction regimen achieves the highest CR/CRi rates is unknown, but this is important, as CR is a prerequisite for allo-SCT. We use standard 7 + 3 or often try to incorporate cladribine, such as in the CLAG-M regimen (R. Tibes, personal communication). Of course, a clinical trial should be considered if patients qualify. Achievement of a CR/CRi or possible second chronic phase in conjunction with allo-SCT is essential for long-term survival, and the potential for cure with allo-SCT.
Lower-intensity therapies
Hypomethylating agents have shown some activity in MPN-BP patients who are not induction- and/or transplant-eligible. The largest study from the French group has reported on 54 patients with either LT after primary myelofibrosis or post-ET/PV myelofibrosis (26 of the 54 patients) or development of a myelodysplastic syndrome/MPN clinical picture (28 of the 54 patients). All patients were treated with azacitidine. At a median follow-up of 20 months, the overall response rate (CR + CRi + partial response) for MPN-BP patients was 38% (10/26 patients), with only 2 CRs; the median overall survival was 8 months for the MPN-BP patient group treated with azacitidine.20 Other smaller case series have shown the activity of decitabine in MPN-BP, with a median survival of greater than 9 months from time of LT, as well as symptomatic improvements and spleen size reduction.21 However, it should be noted that this latter is a case series which included only 6 patients. Thus, while hypomethylating agents have activity, their overall experience and overall benefit in MPN-BP are still limited. Nevertheless, for MPN-BP patients who are ineligible for induction chemotherapy and/or allo-SCT, a default lower-intensity treatment option can be hypomethylating agents alone or preferred combination on a clinical trial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree