4
Irreversible Electroporation Ablation:Mechanism of Action and Devices
The process of electroporation dates as far back as the mid-1700s.1 Only recently, however, has the biomedical potential of irreversible electroporation been considered for various applications including tissue ablation. It was in the 1980s that the term electroporation was coined.1 Electroporation is a technique that uses an electric field to produce a transient disruption of the lipid bilayers of the cell membrane. Under the electric field, membranes rearrange themselves to form temporary pores through which intracellular contents can freely communicate with the extracellular environment.2 The electric field also provides a local driving force that propels larger or polar molecules and ions, which the membrane would normally be impermeable to, into the cell. This technique, reversible electroporation (RE), uses relatively low energies and has been used to introduce a variety of vectors into cells, ranging from DNA and viruses to macromolecules and enzymes.2 RE does not induce direct cell death; the alterations in membrane permeability are transient. Irreversible electroporation (IRE) is the use of the same concept to permanently create innumerable heterogeneous nanoscale pores in the cell membrane by exposing cells to notably higher electric energies.2 Conversely, the altered intracellular environment ultimately induces cell death via both apoptosis and coagulative necrosis.2 IRE has gained increasing attention in the field of interventional oncology as a new minimally invasive ablation technique. This technique is fast and easy to apply, does not require the use of adjuvant drugs, does not cause damage to the vasculature, and is not affected by local blood flow, making it a very specific method of tissue ablation with great potential for cancer therapy.
♦ The Science of Irreversible Electroporation
Although RE has emerged as a useful methodology in biomedical technology and in medicine over the past three decades, there has recently been a shift toward the use of IRE for tumor ablation. Similar to RE, in IRE a strong external electric field is applied to cells to increase transmembrane potential and induce the formation of permeable pores in the membrane. Permeabilization occurs only when the potential difference exceeds a critical threshold value that depends on cell type and is usually between 200 and 300 mV/cm.3,4 Unlike RE, however, the electric pulse strength and duration in IRE surpasses the threshold value to permanently damage the plasma membrane.5 This ultimately leads to cell death because the membrane is unable to return to its homeostatic status, and the nanoscale pores induced by the electric field are permanently opened.6
Cells are highly compartmentalized with a lipid bilayer plasma membrane that regulates intra- and extracellular solute transport. When an external direct current electric field is applied to a cell, it passes around the cell and not directly through it.7 This makes the intracellular current density less than the extracellular current density, creating a voltage potential difference across the plasma membrane of the cell.8,9 When the transmembrane potential hits a certain voltage threshold, the cell becomes unstable and the plasma membrane undergoes breakdown or structural rearrangement to create nanoscale pores in the membrane; thus, electroporation is observed.8,10 Once the membrane is permeabilized, the cell must put in an increasing amount of energy to maintain the transmembrane ionic concentration differences. Conductance of the plasma membrane shoots up in value and if the adenosine triphosphate–dependent protein ionic pumps are unable to compensate for the diffusion of ions through the pores in the plasma membrane, then the cell becomes energy depleted. The cell will then undergo biochemical arrest followed by cell death.8 The irreversibly permeabilized cells then remain in situ to be removed by the immune system.11
The precise mechanism of cell death in IRE-induced ablation is still under debate. However, both necrosis and apoptosis may play a role in IRE-induced cellular destruction.12,13 Recent data suggest that increased apoptotic markers may be found in post-IRE ablated tissue.12 Apoptosis induces innate cellular regeneration and requires less time for tissue healing and recovery than does necrosis. Additionally, apoptosis causes less fibrosis in the ablated area than does necrosis, which would help prevent further damage to ablated organs. Further studies are needed to elucidate the precise mechanism of cell death in IRE-induced ablation to advance our understanding of IRE and its capabilities.
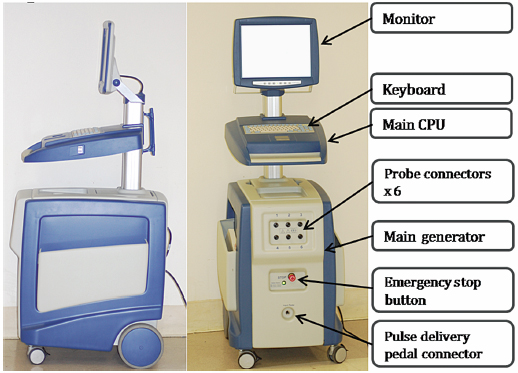
Fig. 4.1 The side and front views of the IRE generator trolley reveal an LCD monitor attached to the main central processing unit, which runs with the Microsoft Windows XP operating system. These two top parts are also connected to the bottom part; the generator harbors six probe connectors and a pulse delivery foot pedal connector.
♦ Ablation Systems
Ablation Equipment and Generator Setup
This protocol is based on using NanoKnife® generators (Fig. 4.1) and probes (AngioDynamics Inc., Queensbury, New York). There are two main types of IRE probes: monopolar and bipolar. The monopolar system (Fig. 4.2) requires placement of two probes into or around the target. The electric current travels between the tips of the two probes to create an electric field, which results in ablation. Each monopolar probe is a 19-gauge needle with a maximum depth of penetration of 15 cm. A 1.5-cm spacing between the two probes with 2500 V results in approximately a 2 × 3 × 3–cm ablation zone. Up to six monopolar probes can be placed simultaneously, allowing for the development of overlapping treatment zones, which can result in a much larger total ablation area. The bipolar system (Fig. 4.3) requires the placement of a single probe. It contains two poles within its distal portion, which creates IRE ablation. The bipolar probe is a 16-gauge needle with a maximum depth of penetration of 18 cm. With 2500 V, it will create approximately a 2 × 2 × 3–cm ablation zone.
The following brief steps are used to set up the IRE/ NanoKnife Generator for any typical solid tumor ablation:
- Boot-up the system. Only the Microsoft Windows XP operating system platform is available at this time. Complete self-testing, including initialization, test charge, and test delivery, is required. If any of these self-testing steps fails, the generator will not function.
- Enter the subject/patient data. The ID number is absolutely required for a log purpose. This automatically generated log is very useful in troubleshooting any technical failure with the generator.
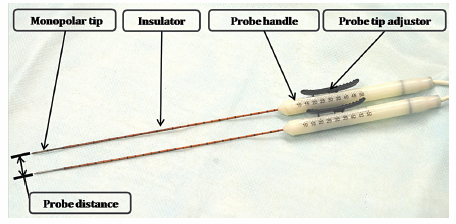
Fig. 4.2 The IRE monopolar probes (19 gauge). Each probe has one polar tip, and the distance between two tips determines the size of the ablation zone. Each probe has an insulator that is connected to the probe handle. Each probe tip can be exposed from 0.5 to 4.0 cm, using the probe tip adjustor to further optimize the size of the ablation zone.
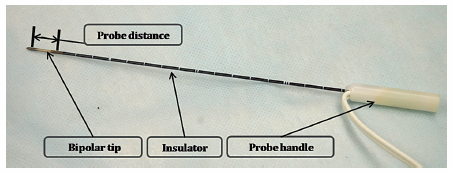
Fig. 4.3 The IRE bipolar probe (16 gauge). Each probe has two polar points and has a fixed distance between the polar points (1 cm). Therefore, the size of the ablation zone cannot be adjusted using the bipolar probe. The bipolar probe also has an insulator portion connected to the probe handle.
- Enter the probe selection: monopolar versus bipolar versus multiple monopolar.
- Enter the treatment parameters (pulse parameters):
- Number of probes
- Voltage (1500–3000 V/cm)
- Pulse length
- Number of pulses = 90 pulses
- Delivery of test pulse
- Delivery of IRE ablation
- Enter the probe selection: monopolar versus bipolar versus multiple monopolar.
Ablation Protocols
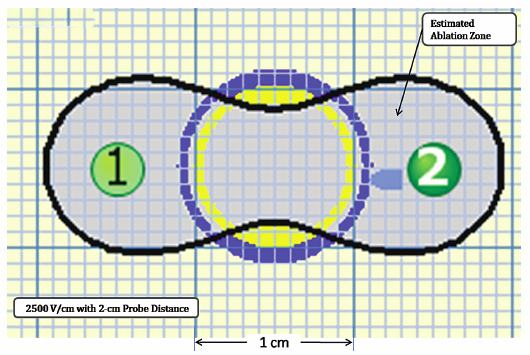
Using the Lesion Estimator (from NanoKnife software version 2.1.0), the following pretreatment protocol can be planned: to ablate a 1-cm-diameter tumor, at least, a voltage of 2500 V/cm with 1-cm spacing between two monopolar probes is required (Fig. 4.4). As can be seen in Figs. 4.5 and 4.6, a smaller voltage or large distance between the probes can create a suboptimal ablation zone. This lesion estimator has been very accurate in predicting the actual lesion sizes in both preclinical and clinical studies. Essentially, any sized tumor can be planned in pretreatment and ablated using IRE, multiple probes, and the IRE lesion estimator.
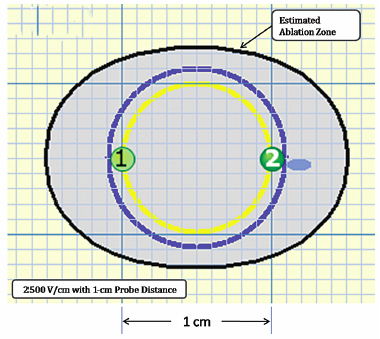
Fig. 4.4 The estimated area of the IRE-ablated zone using 2500 V/cm with a 1-cm probe distance between two monopolar probes. This creates an optimal ablation zone (light blue area) fully covering a 1-cm tumor with adequate tumor-free margins. (Courtesy of Tachi Callas, Senior Research Engineer at AngioDynamics Inc., Queensbury, New York.)
Based on our studies and the manufacturer’s recommendation, we recommend using a minimum electric voltage of 1500 V/cm with at least a 1.7-cm probe distance and 90 pulses for a single ablation session. Depending on the number of pulses, the size of ablation will change accordingly: more pulses will create a larger ablation zone.
Figure 4.7 demonstrates an ablation protocol using a bipolar probe. With a single bipolar probe, it will create an ablation zone of 3.0 × 1.5 × 1.5 cm. To ablate a 1-cm-diameter tumor, it is optimal to place the bipolar probe in the center of the tumor and ablate it once. Any tumor size larger than 1 cm will require more than single ablation session or overlapping of probes.
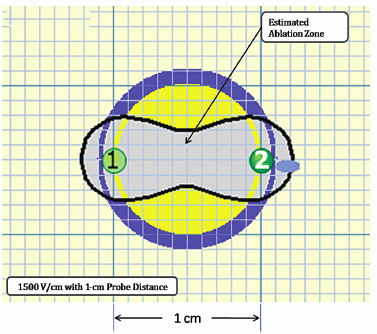
Fig. 4.5 The estimated area of IRE-ablated zone using 1500 V/cm with a 1-cm probe distance between two monoprobes. This creates subop-timal ablation without adequate tumor-free margins, as can be seen in the yellow area. (Courtesy of Tachi Callas, Senior Research Engineer at AngioDynamics Inc., Queensbury, New York.)
Fig. 4.6 The estimated area of IRE-ablated zone using 2500 V/cm with a 2-cm probe distance between two monopolar probes. This also creates a suboptimal ablation zone without adequate tumor-free margins, as can be seen in the yellow area. In addition, this pretreatment planning will ablate large areas of normal tissue. (Courtesy of Tachi Callas, Senior Research Engineer at AngioDynamics Inc., Queensbury, New York.)
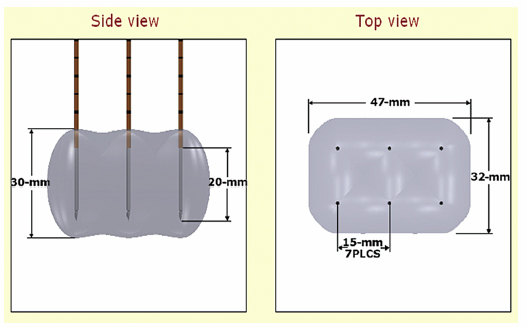
Figure 4.8 demonstrates that a maximum number of probes can be placed simultaneously. Six probes can be placed and ablated concurrently to create a large ablation zone. This ablation protocol will create an ablation zone of 3.0 × 4.7 × 3.2 cm. Using a conventional 90-pulse protocol, the ablation time for using this six-probe configuration is approximately 11 minutes. This is an optimal protocol with which to ablate a tumor with a diameter of approximately 3 cm.
Anesthesia and Procedure Room Setups for Irreversible Electroporation Ablation
Irreversible electroporation uses a high electric voltage to ablate a tumor, and one of the main concerns is a considerable amount of muscle spasm as a result. In this section, we briefly discuss appropriate anesthetic protocols for IRE.
Fig. 4.7 The estimated area of IRE-ablated zone using 2500 V/cm with a single bipolar probe. (Courtesy of Tachi Callas, Senior Research Engineer at AngioDynamics Inc., Queensbury, New York.)
Fig. 4.8 The estimated area of IRE-ablated zone using 2100 V/cm with a 1.5-cm probe distance between six monopolar probes. (Courtesy of Tachi Callas, Senior Research Engineer at AngioDynamics Inc., Queensbury, New York.)
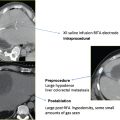
As IRE is performed using ultrasound or computed tomography (CT) guidance, preplanning the placement of all the necessary anesthesia equipment is essential. The anesthesia machine, drug trolley, IRE-NanoKnife generator, ultrasound machine, and defibrillator should be placed in the procedure room once the patient is positioned. The patient should be positioned as per any other ablation or biopsy, and positioning is dependent on the site of the tumor. As for any other CT-guided techniques, special care should be taken to be sure that the patient’s arms are well positioned and secured, and that all the lines are well controlled and placed. Be careful when positioning the IRE not to overextended the patient’s shoulders, which under prolonged general anesthesia can result in brachial nerve palsy.
Standard monitors are required including electrocardiogram (ECG), end-tidal carbon dioxide concentration (ET CO2), blood pressure, oximetry, and temperature. In addition to these standard monitors, the level of neuromuscular blockade should be constantly monitored with a nerve stimulator as all patients require a muscle relaxant such as cisatracurium (trade name Nimbex, Abbott Laboratories). Cisatracurium is an intermediate-acting neuromuscular blockade agent used for both induction (150–200 μg/kg IV × 1) and maintenance (30 μg/kg IV q 20 minutes prn; give the first maintenance dose 40 to 60 minutes after the induction dose). It has a very short onset time of 1.5 to 2 minutes, lasting 55 to 60 minutes, and therefore is the neuromuscular relaxing drug of choice for IRE ablation with a very short procedure time.
In addition to neuromuscular blockade monitoring, an intraarterial line for arrhythmias and the monitoring of regular blood gases are recommended, as some of the IRE-treated patients in the preliminary study in Melbourne, Australia, have shown a mild, transient metabolic acidosis and hyperkalemia. These findings are likely due to cellular destruction of IRE ablation, which did not require any treatment. Lastly, no patients required a Foley urinary catheter for the IRE procedure.
Patients are induced with midazolam/propofol/fentanyl combination as induction agents, with cisatracurium as a nondepolarizing muscle relaxant. The maintenance anesthesia was performed using oxygen/air/sevoflurane. The actual dosage and technique may vary depending on each patient and the location and pathology of the tumor being ablated.
Delivery of a high electric current close to the heart in the treatment of lung lesions or high left liver tumors close to the diaphragm has resulted in arrhythmias, including ventricular fibrillation in preclinical studies and in two patients in the preliminary clinical study. The interventionalist and anesthesiologist in the IRE suite should be aware that the ECG does not provide a reliable tracing during IRE pulses, and therefore an intraarterial pulse/oxygen monitor should be used as a primary source of actual cardiac function during the IRE pulses. Currently, an ECG gating synchronization mode is installed in all commercially available IRE generators, and the issues of arrhythmias may be a thing of the past. Nevertheless, this is still being investigated.
♦ The Advantages and Disadvantages of Irreversible Electroporation
A critical component of IRE is the ability to ablate tissue using nonthermal energy. Previously, it was thought that cellular damage imposed by an electric field was solely a result of the thermal effects of applying an electric field; however, studies have shown that electric fields can also induce tissue damage through IRE, without inducing thermal damage.8,14 This can be achieved by applying ultrashort pulses and large amplitudes of electric current to damage intracellular homeostasis without damaging cellular scaffolding.8 With IRE, the concern was that because higher electric field strengths were needed for IRE than for reversible electroporation, this would cause joule heating and induce tissue ablation primarily through thermal damage and not through IRE. However, recent studies have shown that IRE results in tissue ablation independent of any heat-sink effect (dissipation of heat via adjacent vessels).12,13,15,16 The lack of thermal energy is due to the ultrashort (millisecond) nature of the pulses delivered. In addition, the treatment encompasses multiple short sessions (length of microseconds) rather than one long continuous session. Therefore, the extent of thermal damage becomes negligible. As a result, IRE can cause tissue ablation prior to the onset of thermal tissue damage and protein denaturation.11 To optimize IRE by minimizing increases in tissue temperature, studies have utilized a larger number of short pulses rather than a smaller number of longer pulses, and have also applied delays between pulses to allow tissue cooling.15 Thermal effects are also minimized using more pulses with a lower electric field rather than less pulses with a higher electric field.17
Another advantage of IRE is that it creates complete cell death within the ablated zone regardless of the location within the organ (e.g., in case of liver ablation, interlobular versus intralobular or perivascular versus peribiliary ductal). IRE ablated areas with complete cell death are seen as well as demarcated zones that can be outlined in both macro- and microscopic evaluations. This identifies an important and unique characteristic of IRE, being a potential tumor ablation method that is not affected by the heat-sink effect. This is a significant limitation of thermal ablation, as perivascular tumor can prove extremely difficult to ablate and is the leading cause of recurrence.18 Additionally, IRE appears to cause complete ablation up to the wall of large blood vessels. Because IRE affects only cell membranes and is not affected by blood flow, this allows tumor tissue to be targeted in a very specific and controlled manner while preserving surrounding healthy tissue and scaffolding, where a distinct demarcation is observed between the ablated and the nonablated zone, providing a useful marker of ablation with preservation of surrounding vital structures.12,13,16 Healthy tissue is able to regenerate quickly following ablation because scaffolds are left unperturbed. Other key features of IRE that have been reported include the ability to preserve large blood vessels, bile ducts, and connective tissue in short-term follow-up studies.13 When IRE is applied to blood vessels, all the cells on the margin of the blood vessel are ablated, including the vascular smooth muscle cells. As a result, because the blood vessel matrix remains intact, IRE seems to be a safe and effective method of ablating tumors near large blood vessels without great risk of vascular damage.19
Irreversible electroporation has also been shown to have the shortest procedural times (microseconds to milliseconds) when compared with existing conventional thermal ablation techniques. IRE requires an actual ablation time of less than 1 minute to complete ablation of a 3-cm diameter lesion in the liver. This is a significant decrease in procedure time when compared with other conventional ablation methods that usually take from 10 minutes to a few hours.12 As a minimally invasive ablation technique, with a short procedure time, IRE may provide patients with faster recovery times, a lower number of complications, and a better overall tumor treatment experience than conventional tumor ablation methods.12
The ability to use real-time monitoring with ultrasound is an important advantage of IRE ablation technology. Physicians have the ability to use real-time ultrasound imaging starting with the insertion of the probes, continuing with the IRE ablation, and ending with postablation surveillance to check for any complications. A hypoechogenic area viewed on ultrasound during IRE-induced tissue ablation is an indication of ablated tissue. This is seen immediately on ultrasound and correlates directly with the rough estimate of the size of the ablated lesion.12 If the entirety of the tumor is not fully ablated, with real-time monitoring additional IRE-induced ablation can be performed to ensure complete tumor eradication, greater procedural efficacy, and improved recovery time.
Although IRE has several advantages, one of the primary disadvantages of electroporation results from its fundamental reliance on electric fields. In irreversible electroporation, high-voltage electric fields must be applied, and this can have important effects on the heart. This may be important in IRE because the amplitude and duration of the electric pulse is greater than in reversible electroporation. The period of greatest risk of inducing arrhythmias from the application of an electric field occurs during atrial or ventricular systole.20 Functional abnormalities, most commonly seen in the elderly, who make up the largest pool of IRE patients, can increase the risk of ventricular fibrillation caused by an applied electric field. However, in a recent study of epicardial ablation with IRE, no permanent arrhythmias or heart rhythm disorders were observed as a result of the electric pulsations applied.21 A recent study of the effects of electroporation pulses on the heart indicated that even when IRE pulses are applied directly above the heart, no effect on the functioning of the heart can be detected.22
Despite these observations, to ensure the safety of the patient, it is best to gate the electric pulses with the electrocardiogram to decrease the risk of electric pulse effects on the heart. In addition, as discussed above, one of the main concerns in performing IRE is the considerable amount of muscle spasm that the patient may experience during the procedure. Muscle relaxants (cisatracurium), therefore, are used when performing IRE ablation to prevent electric pulse-induced muscle contractions. The drawback of using muscle relaxant is that extra caution is needed at the end of anesthesia, as the airway can easily collapse if not monitored properly.
- IRE is a novel, nonthermal, tumor-ablative method that is not affected by the heat-sink effect.
- IRE is fast, safe, and potent.
- IRE has real-time monitoring capability using ultrasound.