7
Renal cell carcinoma (RCC) exhibits unique epidemiologic and pathophysiologic characteristics compared with most other neoplasms. It is mass forming (not infiltrative), usually small at time of diagnosis (approximately 70% of newly diagnosed RCCs are <4 cm), and involves an organ that is duplicated and surrounded by retroperitoneal fat. Additionally, it is slow growing, with a reported doubling time of 500 to 600 days, the risk of metastatic disease is correlated to the size of the primary tumor and thus usually is a late-stage complication, and is easily detectable by current imaging modalities. These factors conspire to render most patients with RCC potentially curable. Because of the drive to preserve as much normal renal parenchyma as possible, the role of percutaneous ablation has been expanding; however, treatment selection is occasionally not straightforward.
♦ Current Treatment Options: Placing Ablation into Context
There are several choices for the treatment of organ-confined renal cancer, including percutaneous ablation, open ablation, open partial nephrectomy, open nephrectomy, laparoscopic nephrectomy, laparoscopic partial nephrectomy, and laparo-scopic ablation. Total nephrectomy, whether open or lapa-roscopic, should be a choice of last resort. Nephron-sparing interventions are the current gold standard. Partial nephrectomy, when technically feasible, has been shown to have the same long-term efficacy as that of total nephrectomy, and this is the treatment most commonly selected. Whether to perform open or laparoscopic partial nephrectomy depends on tumor morphology and operator experience. Percutaneous ablation, building on laparoscopic or open ablation safety and efficacy data that are comparable to those of nephron-sparing surgical options, has carved an expanding niche. Initially reserved only for patients who were not surgical candidates, percutaneous ablation is a very attractive option for patients who wish to avoid surgery. This shift has been partly fueled by the ability of patients to use the Internet to better understand treatment options and by the fact that possible failure of percutaneous ablation does not obviate surgical options.
Radical nephrectomy should be a choice of necessity and selected only if a curative nephron-sparing treatment option is not available. This is usually the only treatment option for large, central renal masses in which adequate surgical margins cannot be obtained without sacrificing the main renal artery or vein. Given the faster recovery and fewer complications in experienced hands, laparoscopic nephron-sparing options are preferred. Those include laparoscopic partial resection and laparoscopic ablation. During the last few years, several authors published their experience with percutaneous ablation for RCC. Despite the lack of prospective randomized studies, the fact that these independent authors report near identical safety and efficacy rates corroborates the utility of percutaneous ablation. For lesions 4 cm or smaller, the reported efficacy hovers around 95% and the risk of significant complications around 6%.1 Even though these numbers compare favorably with the other nephron-sparing options, long-term data are still lacking.2,3 Precisely because RCC is a slow-growing tumor, 1- and 2-year data cannot be interpreted as conclusive, and therefore 5-year data are necessary. Nonetheless, a significant number of patients can benefit from percutaneous ablation.
♦ Patient Selection
Occasionally the treatment choice will be clear. For example, a large RCC in a healthy patient with normal kidney function and without high risk for metachronous occurrence should be removed surgically, preferably avoiding nephrectomy. At the other end of the clinical scenario spectrum, are patients who cannot or should not have surgery for their small RCC. Such patients include high-risk anesthesia/surgery patients, patients with solitary kidney predialysis patients, patients with bilateral tumors, and patients with syndromes that predispose them to metachronous RCC. These lesions generally should be treated with nephron-sparing interventions, including percutaneous ablation. Because of the emerging treatment options and the need to preserve renal function even in patients with two well-functioning kidneys, there is a large gray zone in between. This is further confounded by the fact that many patients drive their own treatment toward nonsurgical options, especially because a possible failure of percutaneous ablation does not exclude the treatment the patient would have had in the first place. Whatever the case may be, the decision on the most appropriate treatment option should be arrived at after consultations with specialists who cover the treatment spectrum, including urologists and interventional radiologists.
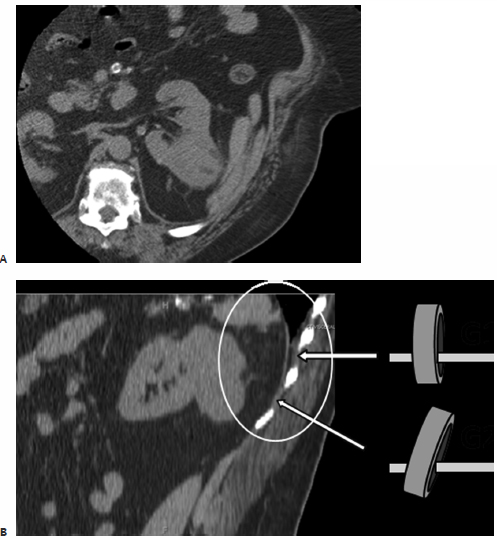
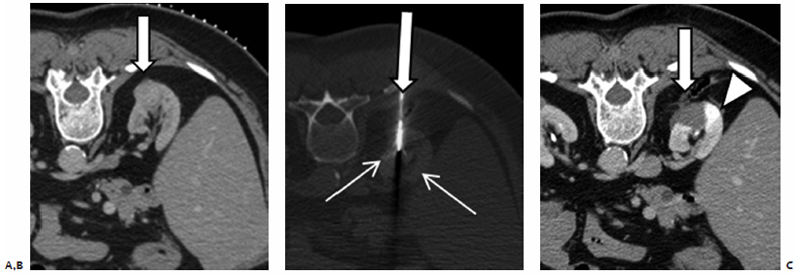
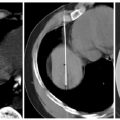
Fig. 7.1 (A) Axial computed tomography (CT) of the left kidney during percutaneous ablation. The renal cell carcinoma (RCC) shows as a large exophytic posterolateral mass (asterisk) and appears easily accessible. Note the small perinephric hematoma (arrowheads) from probe placement). (B) On sagittal reconstructed images, a perpendicular approach (gantry position G1) appears to cross the pleura. Angling the CT gantry caudally (gantry position G2) shifts the access site one rib lower avoiding the pleura. Due to the large size of the mass (patient was not a surgical candidate) the ablation procedure was staged in two visits.
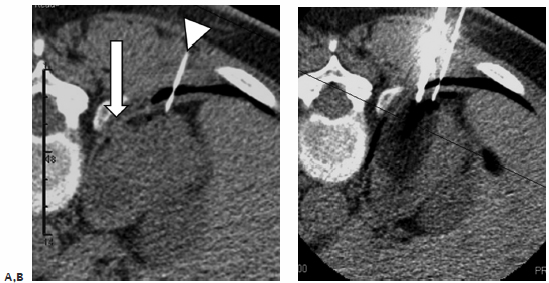
Fig. 7.2 (A) Axial CT image prior to ablation of a small exophytic RCC abutting the diaphragm (arrow). A thin spinal needle (arrowhead) was inserted transpulmonary and air injected in an attempt to separate the mass from the diaphragm. (B) During ablation, the probe is seen in the region of the mass running parallel to the dissection needle, also using a transpulmonary approach. Occasionally, crossing the pleura will be unavoidable, especially for upper pole lesions. This is not a contraindication to the procedure; however, the expertise and equipment for chest tube placement should be readily available.
♦ Tumor Selection
Two factors are the main determinants of the technical feasibility of percutaneous ablation: tumor size and tumor location. Current data appear to support the reported high efficacy and low complication rates of percutaneous ablation, for RCCs that are 4 cm or smaller. Some authors have published very good results in treating lesions larger than that; however, the small number of patients that fall in this category does not allow for definitive conclusions. Tumor location can be a multifaceted challenge. The lesion can be very central, abutting the main renal vessels or collecting system. It can be anterior to the kidney, not allowing for an access window, or it can be adjacent to a vital organ. The operator’s experience is very important in this case. For example, a seemingly untreatable lesion because of location may be successfully approached if the adjacent bowel is pushed away with saline injection. Or, a lesion abutting the collecting system can be ablated safely after the preventive placement of a nephroureteral stent. The following section details these scenarios.
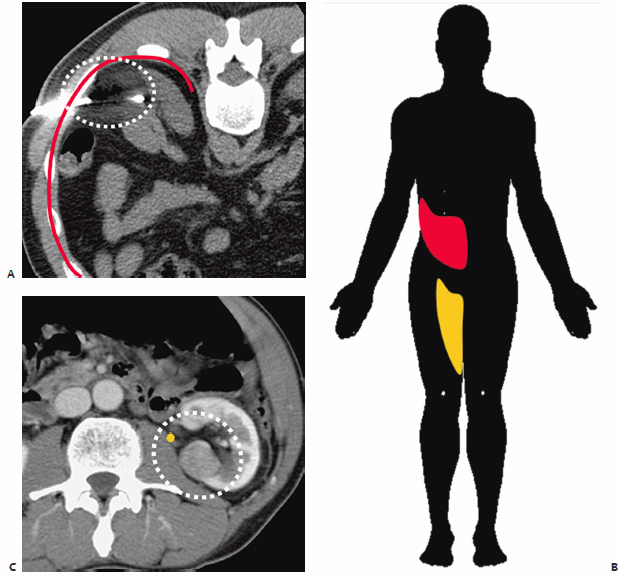
Fig. 7.3 (A) Axial CT image during cryoablation of a posteriorly exophytic RCC. The ice ball is outlined with a dashed white line and encompasses a segment of the anterior division of the intercostal nerve running along the inferior rib margin (red line). (B) Injury to this nerve would result in paresthesias and pain along its dermatome, highlighted in red, and weakness in the related musculature. The associated dermatomal defect is indicated in yellow. (C) Axial CT image of a medial RCC in proximity to the genitofemoral nerve ( yellow), which runs craniocaudally along the anterolateral surface of the psoas muscle.
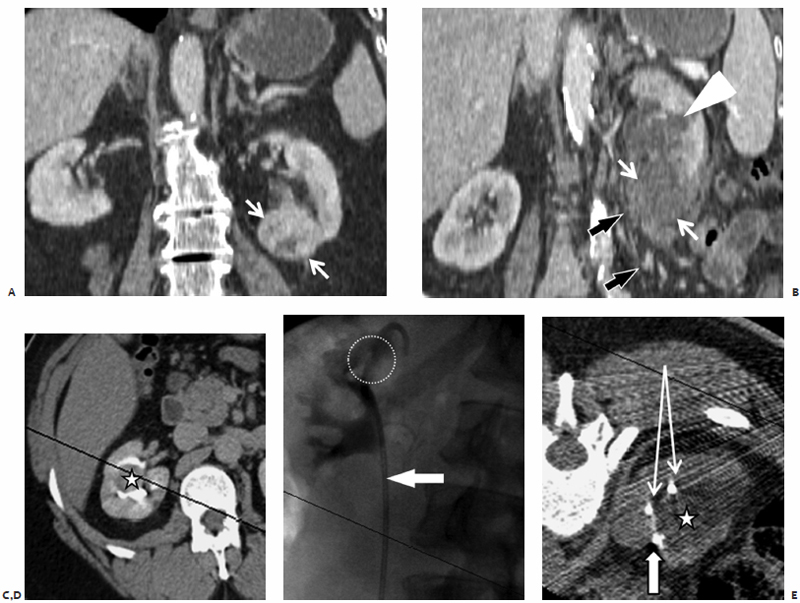
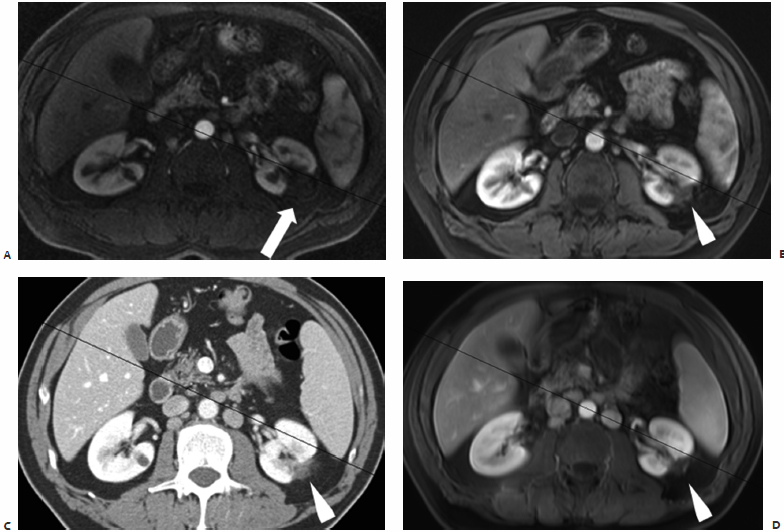
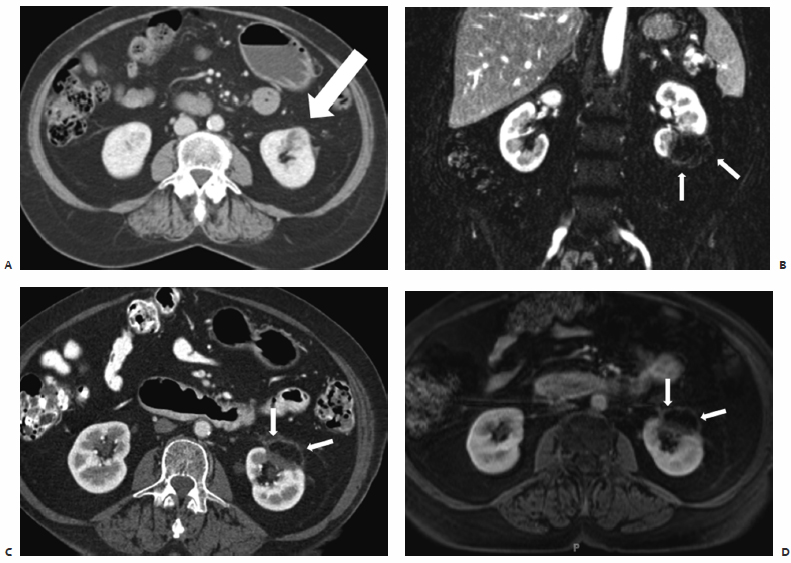
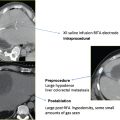
Fig. 7.4 (A) Coronal reconstructed CT image of a patient with RCC shows a 3-cm enhancing mass in the inferior pole of the left kidney (white arrows). The patient underwent uneventful ablation of the mass. A few days later she presented with flank pain. (B) Coronal reconstructed CT image shows a nonenhancing mass corresponding to the ablated lesion in A. Moderate hydronephrosis was also noted (arrowhead), resulting from proximal ureteral scarring, a complication of the ablation. The ureter is seen draping along the anteromedial surface of the lesion (black arrows). The patient required chronic percutaneous nephrostomy drainage. (C) Axial excretory phase CT of another patient shows a central 2-cm RCC nearly completely surrounded by the collecting system. (D) Anterior fluoroscopic view after internal double-J catheter placement shows the catheter (arrow) crossing the collecting system at the location of the mass (dashed circle). This was placed to protect the collecting system from ablation injury, or at least to allow it to heal without obstruction in case of injury. (E) Axial CT image during cryoablation shows the mass (asterisk) barely visible within the ice ball. The tip of the ablation probe is indicated by the solid arrow, whereas cross sections of the double-J catheter are indicated by the thin arrows. The double-J catheter was removed 5 weeks postablation, and there was no evidence of obstruction.
♦ Complications and Anatomic Considerations
Transpulmonary Approach
Occasionally the tumor resides in the upper pole of the kidney, making a transpulmonary approach possible or even necessary. This is not a contraindication to treatment. Figures 7.1 and 7.2 describe such cases. Angling the computed tomography (CT) gantry caudally or using caudal access angle on ultrasound guidance could help avoid crossing the pleura. When a transpulmonary approach is unavoidable, it may result in complications, including pneumothorax or hemothorax. The operator should be capable of recognizing and treating these promptly. If the diaphragm is abutting the target lesion, air dissection or hydrodissection should be used to prevent injury. Though rare, diaphragmatic rupture requiring surgery has been reported, and is more likely to be seen with radiofrequency ablation (RFA) than with cryoablation.
Nerve Injury
There are two nerves that are at risk of injury during percutaneous renal ablation (Fig. 7.3), the first being the lower intercostal nerves that run along the undersurface of each rib. Injury to one of these nerves can cause both motor and sensory deficits. Motor deficits usually go unnoticed because the related muscles (external intercostal muscles, obliquus externus abdominis, transversus abdominis, and obliquus internus) have cross-functionality with other muscles, or the deficit is small. On the other hand, sensory deficits are frequently encountered and include paresthesias and pain along the dermatome. The second is the genitofemoral nerve that runs craniocaudally along the anterolateral surface of the psoas muscle. This nerve is at risk of injury when peripheral lesions on the medial surface of the kidney are targeted. Injury to this nerve results in sensory findings along the inner thigh including pain, numbness, and paresthesias. There are reports asserting that cryoablation is more forgiving than heat ablation, especially related to nerve injury. Indeed, permanent nerve injury after cryoablation is rare, and most patients tend to recover within weeks of nerve injury.
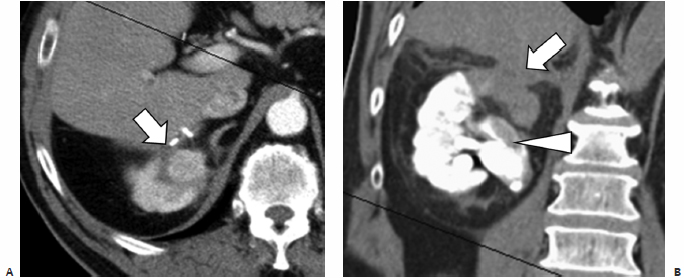
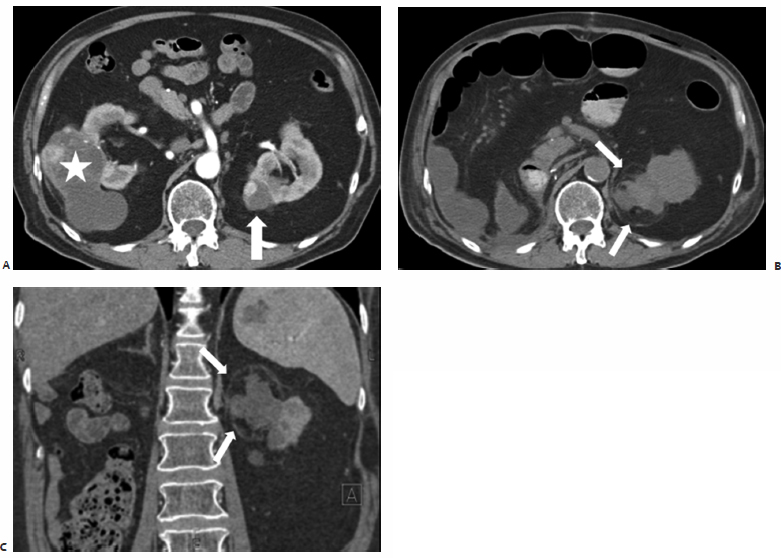
Fig. 7.5 (A) Axial contrast enhanced CT image shows a 2.5-cm enhancing RCC (arrow) in the upper pole of the right kidney. The patient had open cryoablation 3 years prior, and presented with locally recurrent disease. CT-guided percutaneous cryoablation was performed successfully; however, during the recovery period the patient developed myocardial ischemia and underwent emergent coronary angiography. A tight right coronary artery stenosis was uncovered and treated with a stent necessitating Integrilin, Plavix, and aspirin administration. The patient developed significant hematuria. (B) Coronal reconstructed unenhanced CT image shows a delayed nephrogram (from coronary angiography) and hydronephrosis from collecting system thrombus (arrowhead). The ablated mass and margin (arrow) do not demonstrate a delayed nephrographic picture because at the time of coronary angiography the area was already devascularized from the ablation. An internal double-J ureteral stent was placed to prevent renal failure due to obstruction.
Collecting System Injury
Ablation of central lesions that abut portions of the collecting system may result in segmental or complete obstruction (Fig. 7.4). Calyceal injury may result in stricture and subsequent obstruction of said calyx, which, however, is unlikely to alter renal function. Another possible consequence of collecting system injury is hematuria. Central lesions are at higher risk; however, in most cases hematuria is not clinically significant and is self-limiting, resolving over the following 2 to 4 days. If the patient is able to void and vitals are stable, no intervention or further hospitalization is necessary. Rarely hematuria can be clinically significant, and results in hematocrit drop or even collecting system thrombus and obstruction (Fig. 7.5). Nevertheless, it may result in a urinoma as the system attempts to decompress, which could be further complicated by infection. Anterior, lower pole lesions represent a special case because of their proximity to the ureter. The ureter drapes along the anterior portion of the lower pole of the kidney. Inadvertent nontarget ablation of the ureter may result in complete obstruction, severe hydronephrosis, and possible kidney loss. Postablation ureteral strictures are notoriously difficult to treat, and the placement of a nephro-ureteral catheter through the stricture may not be possible. The preablation placement of a nephroureteral stent may alleviate this complication. The internal nephroureteral stent is placed just prior to ablation and kept long enough to allow for healing of the injured ureter (at least 4 to 6 weeks).
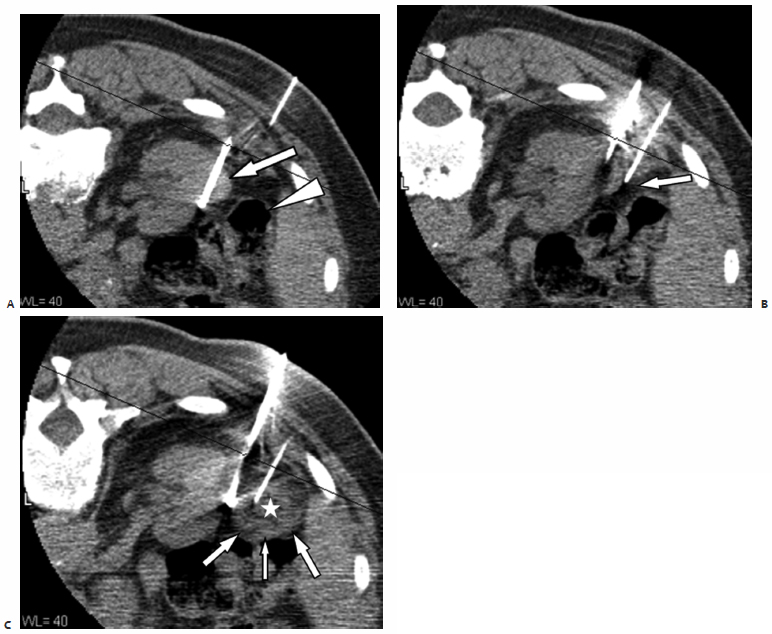
Fig. 7.6 (A) Axial CT during ablation shows the ablation probe transgressing the base of a small, partially exophytic mass (solid arrow). The colon (arrowhead) was close to the mass, increasing the risk for colon injury during ablation. (B) A 22-gauge needle with its tip placed between the target lesion and the colon (arrow). (C) After slow injection of approximately 20 cc of sterile H2O, the colon surface (arrows) is seen being pushed away by the iatrogenic collection (asterisk). Once the distance is deemed adequate, ablation is started. Frequent checks during the ablation are necessary to ensure that the safety margin is maintained. Additional injections may be necessary.
Adjacent Organs
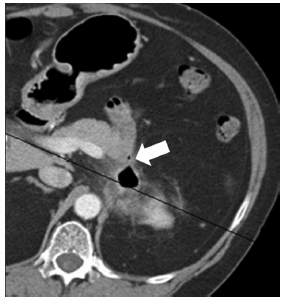
Various organs may be at risk for nontarget ablation. They include the liver, spleen, adrenal, pancreas, and more, frequently, the bowel (Fig. 7.6). Partial nontarget ablation of the liver or spleen is mostly inconsequential, and should not be a contraindication to performing the procedure. Care should be exercised to avoid unnecessarily puncturing these organs, however, because of the risk of hemorrhage. The intentional transhepatic approach has been described and sometimes may be necessary for anterior lesions in the right kidney (Fig. 7.7). RFA offers the added advantage of cauterizing the tract, thus mitigating potential hemorrhage, as opposed to the larger caliber cryoprobes. Nontarget ablation of the bowel (Fig. 7.8) or pancreas, however, may result in serious or life-threatening complications. Certain maneuvers (described later in this chapter) could mitigate this risk and allow the procedure to go forward. However, the inability to avoid ablating the pancreas or bowel should be a contraindication to the procedure. Targeting upper pole exophytic lesions may result in partial or total ablation of one of the adrenal glands. Because ablation of RCC is considered a curative procedure, as long as the patient has two functioning adrenal glands, the risk of partial or even total ablation of one should not be a contraindication to the procedure. Rarely, intentional adrenal ablation may be necessary (Fig. 7.9) as the RCC involves the adrenal gland, by direct extension or as a solitary metastasis. Ablation of a small-enough tumor despite adrenal extension or involvement (stage 3a, N0,M0) (Table 7.1) can still be a curative intervention as long as the rest of the tumor is confined within Gerota’s fascia. The operator, however, should protect against arrhythmia or a hypertensive crisis as a result of catecholamine release during adrenal injury. Beta- as well as alpha-blockade may be required prior to ablation.
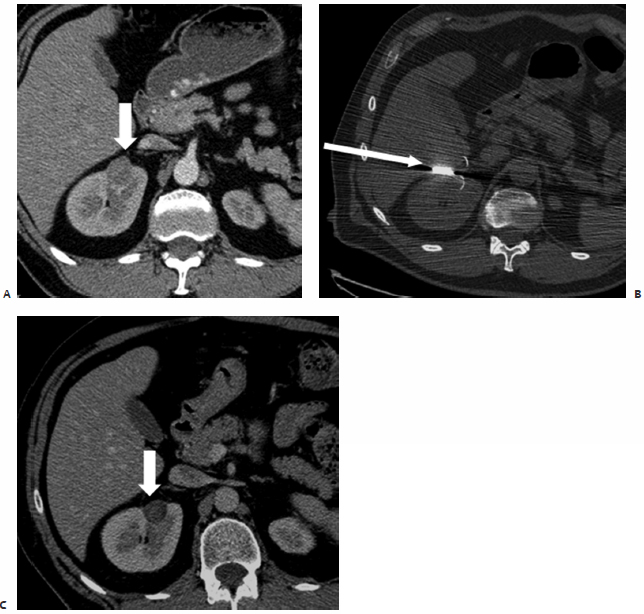
Fig. 7.7 (A) Diagnostic axial CT image during the venous phase of a contrast-enhanced study shows a 2-cm exophytic RCC on the anterior surface of the right kidney (arrow). (B) An intraprocedural CT image shows a 3.0-cm umbrella type probe (arrow) deployed in the tumor. The tip of this type of probe is placed at the distal portion of the mass because the tines expand along a lateral and posterior direction. A trans-hepatic approach was deemed necessary. The lesion could have been approached using a transrenal posterior approach; however, the risk of hemorrhage is greater with renal injury compared with hepatic injury. (C) One-month follow-up contrast-enhanced CT shows a completely devascularized and partially shrunk mass (arrow). (Courtesy of Dr. Paul Harrod-Kim.)
Fig. 7.8 Axial CT image on postoperative day 4 after RFA of a left mass. Patient presented with fever and an elevated white blood count. The ablated necrotic tumor appears to have liquefied and become infected, as indicated by the presence of gas. The third portion of the duodenum appears kinked/tethered to the necrotic region, and there is evidence of a fistula (arrow). Nontarget ablation likely resulted on duodenal injury and the formation of a contained abscess within the necrotic mass. Broad-spectrum antibiotics and a surgical consult are necessary if bowel injury is suspected.
Anterior Lesions
Anterior lesions are challenging, as they are more likely to be adjacent to nontarget organs and, most importantly, not avail themselves of direct access. The direct transhepatic approach is acceptable if no other option exists for anterior right renal lesions (Fig. 7.7). Occasionally, though less desirable, a direct transrenal approach is necessary to treat such a lesion (Fig. 7.10). This increases the risk of perinephric hemorrhage, and the patient should be monitored more aggressively postablation.
♦ Technique: A Step-by-Step Approach
Patient Preparation
As with any case requiring conscious sedation, an 8-hour fasting period is required prior to ablation. A normal coagulation profile must be documented. There is no evidence that supports the use of premedication with antibiotics for uncomplicated cases, although there is also no evidence that it does not help prevent infections. The decision is up to the surgeon. Table 7.2 lists the contraindications to percutaneous ablation.
Planning
Preprocedure planning is of paramount importance to minimize potential complications and maximize efficacy. Before the patient is placed on the table, the surgeon must already know the positioning of the patient, the access site(s), the number of probes required, and whether additional maneuvers will be required, such as thermal monitoring or non– target organ protection.
Positioning/Preparation
The patient is placed prone, with the arms above the head to minimize beam hardening and scatter artifact. If the lesion is on the lateral aspect of the kidney, an ipsilateral elevated position may be helpful. After positioning, a preprocedure dry CT is obtained through the region of interest, and the access site/angle is confirmed. This is necessary because on many occasions the relative anatomy of the kidney and adjacent organs may shift with the patient’s being prone (Fig. 7.11). Once the access site/angle is confirmed, the region is prepped and draped in a sterile manner. For lateral horizontal approaches, the patient may have to be shifted laterally to allow enough room in the gantry for probe placement.
Sedation/Local Anesthesia
The choice of sedation is mostly dependent on the type of ablation. Cryoablation does not require general anesthesia, as freezing the tissue is generally painless. On the other hand, RFA can be quite painful, especially in the kidney. Depending on available ablation margins and the patient’s tolerance, general anesthesia may be required for RFA. Local anesthesia is always helpful and should be used generously along all probe access sites even in the case of general anesthesia.
Testing the Probe/Regulator (Cryoablation)
The gas tanks are connected to the regulator and the valves are opened. The probe tubing is also connected to the regulator, which is switched on. The probe tip is placed in a bowl containing sterile water or normal saline and set to freeze for a few seconds. This is to test that the system is indeed functioning and that there is no gas leak from the probe shaft.
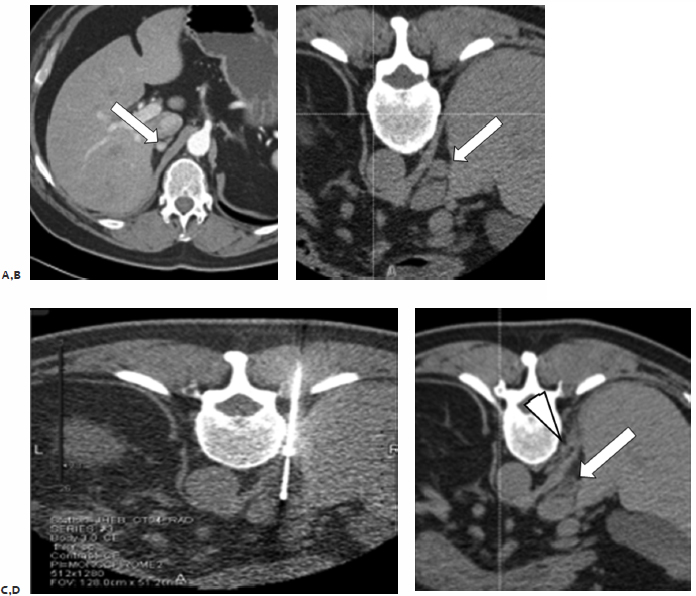
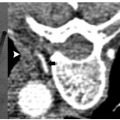
Fig. 7.9 (A) Axial contrast-enhanced diagnostic CT image shows a 0.8-mm enhancing nodule in the right adrenal gland. The patient had undergone total nephrectomy 2 years earlier for organ-confined RCC. The adrenal lesion was a new finding and highly suspicious for recurrence. Patients with RCC often benefit from cytoreduction or metastectomy, especially in cases of limited tumor burden or a single lesion. (B) Axial CT image just prior to ablation shows the adrenal nodule (arrow) in proximity to the inferior vena cava. (C) A paravertebral approach allowed proper probe placement, obviating a transhepatic approach. The ice ball is seen engulfing the adrenal nodule. (D) Immediate postablation CT image shows the adrenal nodule (arrow) still partially frozen and no evidence of the inferior vena cava injury. A small retrocrural hematoma (arrowhead) is noted, which resulted in referred ipsilateral shoulder pain.
Placing the Grounding Pads (Radiofrequency Ablation)
Available heat ablation systems utilize radiowave and microwave frequencies. The former requires the placement of grounding pads to close the circuit, of which the patient is part. The four pads should be placed equidistant from the ablation target to allow for maximum energy dispersion and avoid skin burns.
Access and Probe Placement
Once the skin is anesthetized, a tiny skin incision is made with a scalpel. The tip of the ablation probe is placed superficially in the incision and the probe is aligned with the gantry-mounted laser light. A single CT-fluoroscopy image shows the relative distance and angle of the probe to the target lesions. The probe is gradually advanced under intermittent CT-fluoroscopy visualization. Readjustment of probe direction/depth is necessary during insertion. The exact placement of the probe depends on the probe type, and the operator should consult the manufacturer’s instructions. For example, in the case of cryoablation, the ablation margin does not extend more than 1 to 2 mm beyond the tip; therefore, the tip should be placed at the distal tumor margin. Similarly, for umbrella-type RFA probes, the tip should also be placed distally in the tumor and the tines deployed (Fig. 7.7). On the other hand, for forward-deployed RFA probes the tip of the probe is placed at the proximal tumor margin and the tines deployed distally (Fig. 7.12).
| Stage | Description | Ablation Potentially Curable (Assuming Approachable Lesion) |
|---|---|---|
| Tumor Stage (T) | ||
| 1a | <4 cm, limited to kidney | Yes |
| 1b | 4–7 cm, limited to kidney | Probably (some data) |
| 2 | >7 cm, limited to kidney | Possibly (scant data) |
| 3a | Perinephric or adrenal extension but within Gerota’s | Yes (<4 cm) |
| 3b | Renal vein or inferior vena cava involvement, rest within Gerota’s | No |
| 3c | Inferior vena cava above diaphragm, rest within Gerota’s | No |
| 4 | Outside Gerota’s | No |
| Node Stage (N) | ||
| X | Cannot be assessed | |
| 0 | No nodes | |
| 1 | 1 node | |
| 2 | >1 node | |
| Distant Metastases (M) | ||
| X | Cannot be assessed | |
| 0 | No distal metastases | |
| 1 | Distant metastases | |
Note: Percutaneous ablation for RCC has been shown to have high efficacy (>90%) and low complication rate (16%). Most of the published series are for RCC £4 cm (stage 1a) and have shorter than 5-year follow up. Nonetheless, because of its lower cost, faster recovery, and fewer complications, many patients opt for this treatment. Data for lesions larger than 4 cm (stage 1b) show a similar efficacy and safety profile; however, they are limited and no conclusion can be drawn yet.
The main difference between RFA and cryoablation is that, whereas RFA probes can be used, repositioned, and reused to cover tumors larger than the ablation zone of a single probe, cryoprobes cannot be repositioned after starting the cryoablation. This is because the frozen tissue “sticks” to the probe; therefore, more than one cryoprobe is used to “cover” a larger or complex shaped tumor.
Ablation
Ablation protocols are designed to ensure complete tissue necrosis within the reported ablation zone. As such, ablation protocols do not depend on tumor pathology, size or location, or number of probes used. Assuming the tumor is adequately covered, each ablation system has a unique protocol. Chapter 1 described the RFA systems in detail. Briefly, RFA uses either temperature or impedance feedback to determine adequacy of ablation. Cryoablation protocols were described in Chapter 2; they are set by the time duration and the number of freeze–thaw cycles. Whatever the ablation modality, a minimum of a 5-mm ablation margin (some authors support a 1-cm margin) is required to ensure complete target tissue necrosis. A larger margin is even more crucial for lung cancer because of its spiculated nature.
Assessment
Assessment of cryoablation is relatively straightforward, as the “ice ball” is easily visible under CT guidance. If a portion of the tumor appears outside the ablation margin (the isotherm that is 5 mm inside the visible ice ball), an additional cryoprobe can be placed to provide the extra coverage needed. The operator should use as many cryoprobes as necessary (barring the risk of “cryoshock”) to ensure complete tumor ablation (Fig. 7.13). An insufficient number of cryoprobes is the most common cause of incomplete ablations. On the other hand, RFA changes may not be visible under CT or even ultrasound (US), and when they are, they do not necessarily correspond to necrotic tissue. The lack of real-time changes under either CT or US does not signify a failed RFA. It is therefore paramount, in the case of RFA, to ensure proper probe positioning prior to ablation.
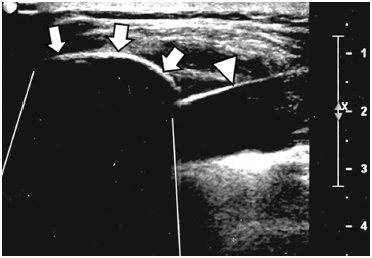
Most renal ablation complications are obvious during ablation. CT guidance offers a better modality to detect these complications compared with US (Fig. 7.14). These complications mostly include perinephric hematoma, pneumothorax, and the risk of nontarget ablation.
Recovery
The intent of postablation patient recovery is twofold: first, to ensure patient comfort by promptly and effectively treating symptoms; and second, to provide an opportunity to detect possible ablation-related complications. Post–renal ablation symptoms commonly involve flank pain, which may range from none to severe, and more rarely ipsilateral abdominal or groin pain (nerve injury), nausea/vomiting, and fatigue. Symptomatic relief is easily achieved with appropriate medications and should not prompt a hospital admission.
Serious complications that can be diagnosed during the recovery period include hemorrhage, pneumothorax, and nontarget ablation. Pneumothorax is easily excluded by a postoperative upright chest radiograph 1 to 2 hours postprocedure. Most pneumothoraces are visible during ablations performed under CT guidance. Nontarget ablation involving the bowel is of grave concern and the operator should be aware of its risk based on bowel proximity during ablation. Peritoneal signs, rigors, and free air under the diaphragm should prompt a hospital admission, CT scan of the abdomen, and urgent surgical consult. Perinephric hemorrhage (Fig. 7.15) can be seen in up to 30 to 50% of renal ablation patients, and most of the time it manifests as a self-limiting perinephric hematoma. Unless it is very large or expanding, or the patient has significant comorbidities, it should not prompt a hospital admission. Postablation hemorrhage is slightly less common with RFA (compared with cryoablation) because RFA cauterizes the damaged vessel(s). Delayed hemorrhage can rarely be encountered especially in patients (re)initiating anticoagulation after ablation.
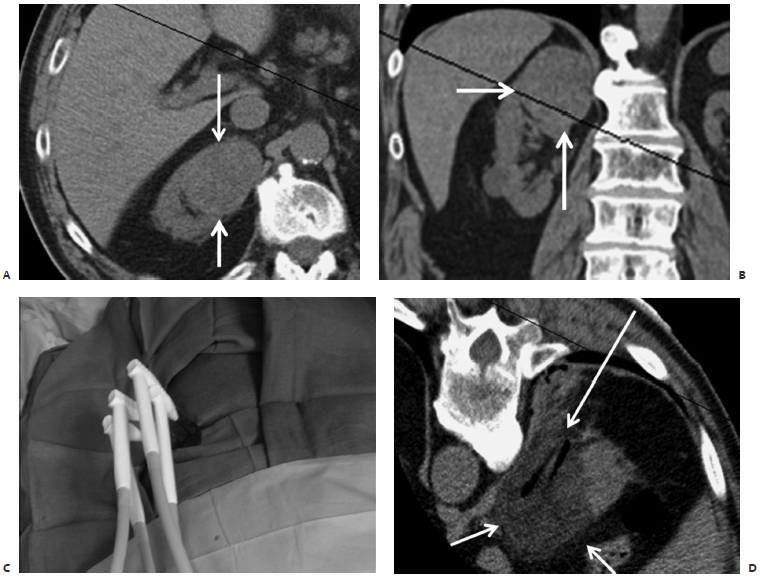
Fig. 7.10 Axial (A) and coronal (B) reconstructed CT images show a large right, upper pole, anterior RCC (arrows). Because of the size and the patient’s comorbid conditions, treatment was staged. (C) During the first ablation four cryoprobes were used and the upper part of the mass was ablated. (D) Axial CT image immediately postablation shows the ice ball (short arrows) covering the mass and the “ghost” of the removed probes (long arrow). Despite choosing a medial, paravertebral approach, one of the probes transgressed into normal renal parenchyma. This may be necessary occasionally, and though it increases the risk of hemorrhage it should not be an absolute contraindication.
A 2-week postablation recovery is recommended before anticoagulation is restarted. This of course has to be balanced against the prothrombotic risks and in coordination with the patient’s internist or cardiologist. In our experience of more than 100 renal ablation procedures, only two patients had hemorrhage requiring intervention. Both were restarted on Coumadin 1 week postablation, presented with delayed hemorrhage, and were managed with blood transfusions alone. Comparing RFA versus cryoablation for renal cell carcinoma, there is an accumulating body of evidence that suggests cryoablation has a greater efficacy and lower retreatment rates than RFA.4
♦ Follow-Up and Postoperative Management
Postoperative management aims to prevent delayed complications and minimize symptoms. A 5- to 7-day course of appropriate antibiotic coverage is commonly prescribed along with pain medications. Other medications can be given according to specific symptomatology (e.g., antinausea medications). Discharge instructions and patient education are important tools that can help minimize the consequences of complications. In general, the worst period as far as patient symptoms is concerned is the same day of the procedure. Worsening or new symptoms after postoperative day 0 should prompt a call from the patient and evaluation.
| Contraindication | Mitigating Action |
|---|---|
| Active infection | Treat and resolve |
| Platelets <50 K | Transfuse platelets |
| INR >1.7 | Transfuse fresh frozen plasma or discontinue anticoagulation |
| NSAIDs within 5 days | Postpone procedure |
| Nontarget vital organ ablation | Air dissection or hydrodissection |
| No percutaneous window | Air dissection or hydrodissection |
INR, international normalized ratio; NSAIDs, nonsteroidal antiinflammatory drugs. Note: Any active infection, not just urinary tract infection, is an absolute contraindication to ablation. Similarly, coagulopathy should be corrected prior to any intervention. NSAIDs slightly increase the risk of periprocedural hemorrhage; however, because percutaneous renal ablation is never an emergency, there should be no reason to rush the procedure. A minimum of a 5-day NSAID-free period, as well as resolution of any infection prior to ablation, is recommended. The lack of an access window or the presence of nontarget organ near the lesion should not be a contraindication to attempting the procedure. Many times, merely by placing the patient in the prone position, the anatomy shifts and allows the procedure to proceed. Air dissection or hydrodissection should address the problem otherwise.
Follow-up varies. Our protocol calls for a 3-, 6-, 9-, and 12-month contrast-enhanced CT or magnetic resonance imaging of the kidneys with noncontrast, arterial, venous, and delayed phase and then annually for life (Figs. 7.16, 7.17, 7.18, and 7.19). Nearly all residual viable tumors will be visible on the first follow-up at 3 months (Fig. 7.20). Simultaneous clinic visits and review of relevant lab findings should also be performed to ensure appropriate patient recovery. No immediate postablation contrast-enhanced cross-sectional imaging is necessary. RCC is a very slow growing tumor, and a 1- to 3-month follow-up is adequate to ensure complete ablation. In addition, clinically nonsignificant findings (Fig. 7.21) can confuse the picture. A non–contrast-enhanced CT can be obtained postablation in cases of suspected, significant hemorrhage.
♦ Tricks of the Trade
Torque, Push, and Pull
During ablation, the ice ball may expand too close to a nontarget organ, risking a serious complication. The probe is frozen stuck into the ice ball, a property that can be used to advantage. By gently pulling (or pushing) the probe, the entire kidney can by moved away from a critical structure. Similarly, gentle torque can be used to shift the kidney laterally or medially, further increasing the distance between the ice ball and the organ at risk for unintentional ablation (Fig. 7.22).
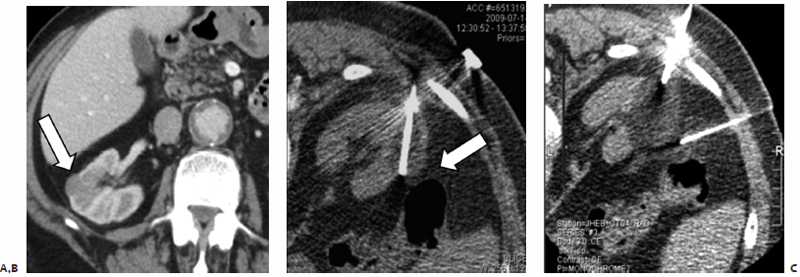
Fig. 7.11 (A) Axial contrast-enhanced diagnostic CT scan shows a 2-cm partially exophytic mass (arrow) from the lateral surface of the right kidney. No critical nontarget organ is noted in proximity to this mass. (B) However, after the patient is placed prone and the probe inserted, the air-filled colon (arrow) appears to have interposed itself between the liver and target lesion. (C) A spinal needle was inserted from a right lateral approach and used to inject saline between the ice ball and colon, pushing the colon away.
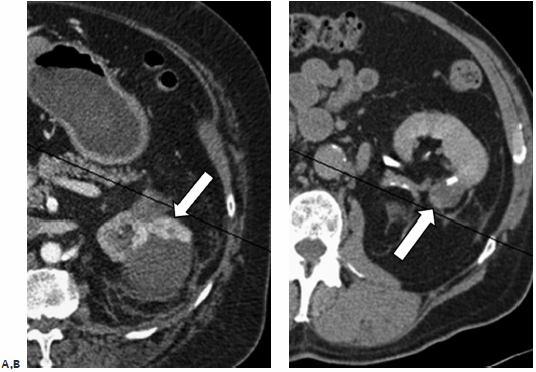
Fig. 7.12 (A) Axial contrast-enhanced CT shows a 2.5-cm posterior right renal mass (arrow). A forward deployed ablation probe was utilized. (B) During ablation, the tip of the probe was placed at the proximal part of the mass and the tines were gradually expanded forward (arrows) until the entire mass and target margin were adequately ablated. (C) Contrast-enhanced follow-up CT scan shows a completely devascularized and collapsed mass (arrow). A small renal parenchymal segment (arrowhead) demonstrates delayed enhancement during this venous phase study, suggesting segmental acute tubular necrosis. No creatinine changes were observed. (Courtesy of Dr. Paul Harrod-Kim.)
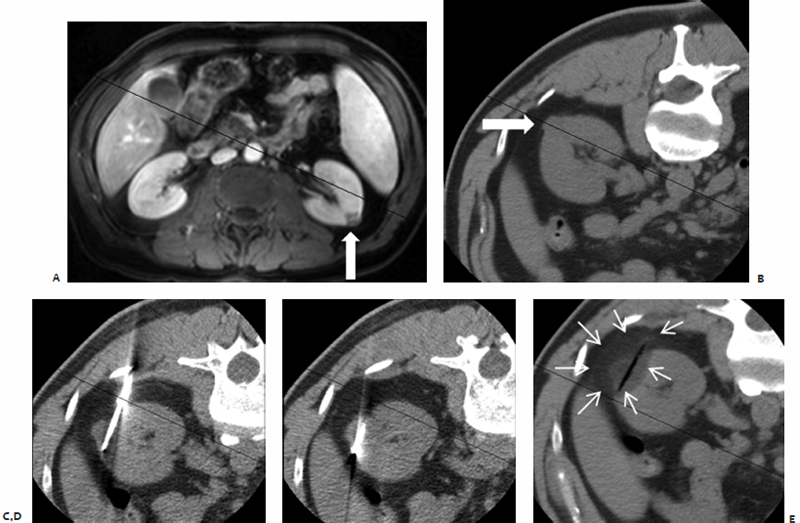
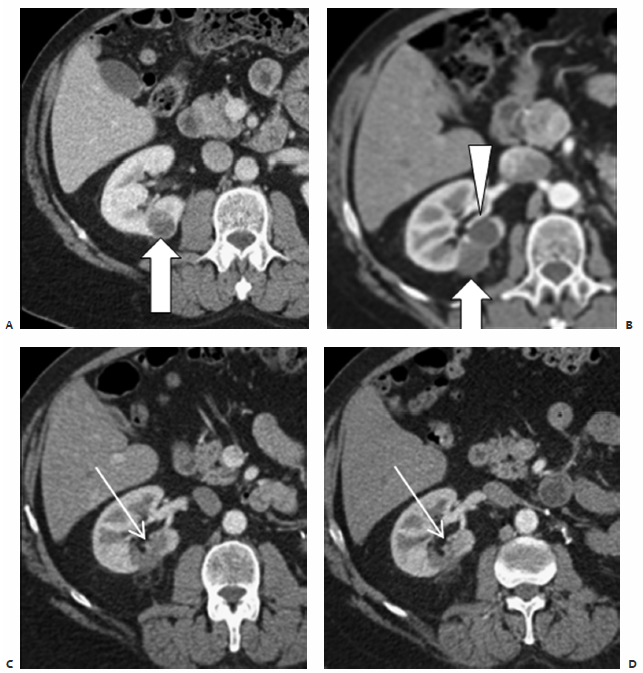
Fig. 7.13 Basic principles of probe placement. The most common cause of residual tumor after ablation is not using an adequate number of probes. Even though a tumor may be barely smaller than the lethal ablation margin of a single probe, complete ablation depends on placing the probe precisely in the center of the mass. In real life this may be very difficult. Outcomes improve if one errs on the side of using more rather than fewer probes. Supine (A) and intraprocedure prone (B) CT images show a 1.2-cm exophytic mass in the left kidney. Even though a single probe will theoretically suffice, the better part of valor is to ensure complete ablation by utilizing two probes. One probe was placed caudally (C) and another cranially (D) through the base of the mass. Care must be taken to ensure adequate ablation of the intraparenchymal portion of the mass. This may not be evident during the ablation because no contrast is used; therefore, planning based on the diagnostic contrast-enhanced study is necessary. (E) The postablation CT shows the ablation zone (arrows) around the probe ghost covering the mass.
Fig. 7.14 Ultrasound image during percutaneous cryoablation. The proximal margin of the ice ball is clearly seen (arrows). However, because the ultrasound waves are mostly reflected back at this surface, not enough sound energy penetrates to interrogate distal structures. The result is a “shadow” that obscures anatomy deep to the ice ball. Possible hematoma or a nearby nontarget organ will not be visible. The cryoprobe shaft is indicated by the arrowhead.
Air Dissection or Hydrodissection?
Solely based on the baseline diagnostic cross-sectional imaging, approximately 10 to 20% of RCCs are deemed not amenable for percutaneous ablation on account of nontarget ablation risk. These adjacent organs, however, can easily be protected with needle dissection. In our unpublished series of more than 100 renal ablations, technical success was 100% with a rate of hydrodissection or air dissection of 16% (Figs. 7.11 and 7.23). Irrespective of how close a nontarget organ is to the target lesion, the procedure should at least be attempted. Many times simply placing the patient prone results in shifting of organs that allows for the ablation to proceed. Air dissection or hydrodissection can be used for the rest. The choice of air or saline use depends on the relative anatomy of the target and the nontarget at risk. Air can shift against gravity, whereas saline shifts with gravity. This can be used to advantage. Finally, it should be noted that for RFA, sterile water should be used in lieu of saline, as the latter conducts electricity. CO2 is less irritating to the peritoneal lining and is absorbed much faster; thus, for large injection volumes it should be preferred over air.
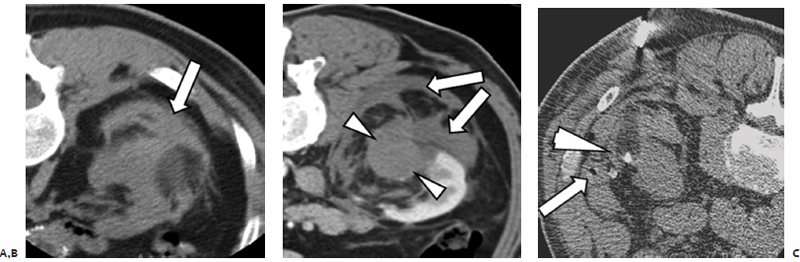
Fig. 7.15 (A) Axial CT image during cryoablation shows a small perinephric hematoma (arrow). Small to medium hematomas are very common after renal ablation (approximately 40%). However, the vast majority are stable and self-limiting, requiring no intervention, and should not by themselves be an indication for admission. (B) CT image of another postablation patient showing a larger perinephric hemorrhage (arrows) and a nonenhancing renal mass (arrowheads). (C) Axial CT image during ablation of a third patient shows a small hematoma (arrowhead). This was a fortuitous occurrence because the hematoma dissected between the target lesion and the colon (arrow), thus allowing the ablation to proceed safely without the need for the operator to inject saline between the two.
Fig. 7.16 Postablation appearance of RCC. Cross-sectional imaging at 3 (A), 6 (B), 9 (C), and 12 (D) months of follow-up shows a wedge-shaped defect (arrow). The sine qua non of a successful ablation is the lack of nodular enhancement and the gradual decrease in size of the mass. Any nodular enhancement or enlargement beyond the 3-month mark is suspicious. The nonenhancing scar tissue (arrowheads), which may or may not be visible depending on the enhancement phase, should not be mistaken for residual tumor. It also gradually decreases in size past the 3-month mark.
Number of Probes, Overlapping Ablations
One of the main technical differences between cryoablation and thermal ablation (RFA, microwave) is that during the latter the same probe can be used multiple times during the same procedure to perform overlapping ablations. This allows an ablation volume larger than the volume possible with one probe alone. On the other hand, because of the solid ice ball, overlapping ablations are not possible with cryoablation. The one exception to this is for longitudinal lesions. If the cryoprobe is placed along the long diameter of the lesion, it can be used to ablate the distal portion first and then withdrawn to ablate the proximal portion. Lateral repositioning, however, is not possible. As a result, for cryoablation multiple probes must be used to ensure adequate tumor coverage.
Gelfoam Embolization After Biopsy
Renal cell carcinomas are notoriously hypervascular, even more so than the very vascular surrounding of normal renal parenchyma. Those who perform RCC biopsies frequently can attest to the fact that significant blood flow can be seen through the coaxial system after the needle is removed. This bleed is a confirmatory sign that indeed the needle was in the RCC; however, it may result in significant retroperitoneal hemorrhage. The main concern is not clinical, because, as we mentioned above, the vast majority of such bleeds are self-limiting, likely due to the lack of potential spaces in the retroperitoneum. The perinephric blood, however, can completely obscure small exophytic masses, making ablation very difficult or even impossible. If back-bleed via the coaxial system is noted, the operator can mitigate its effect by Gelfoam embolization. A Gelfoam slurry is prepared and 2 to 4 cc of thick slurry is slowly injected as the coaxial system is removed.
Fig. 7.17 Postablation appearance of RCC. Baseline (A), 3- (B), 6- (C), and 9- (D) month follow-up CT images of a 1.8-cm RCC (wide arrows in A and B). At 3 months, the mass (arrow in B), though nonenhancing, appears not to have shrunk, and even to have enlarged a bit. This is not an unusual finding, and it does not necessarily signify residual tumor. The determining factor at 3 months is the lack of enhancement. A postablation small cyst (arrowhead in B) is likely the result of partial obstruction. On longer follow-up, the mass gradually decreases in size and more importantly maintains its lack of enhancement, with the pelvic and perinephric fat converging toward the shrinking mass (arrows in C and D). The small cyst has resolved.
Fig. 7.18 Postablation appearance for RCC. (A) Axial preablation CT shows a 2-cm mass in the left kidney (arrow). Three-month coronal MRI (B), 6-month axial CT (C), and 9-month axial MRI (D) all show a thin rim of enhancement around the ablation zone (arrows). This is a very common finding and should not be confused with residual tumor. The rim is beyond the actual tumor and represents a hypervascular scar at the margin of the ablation zone. It may persist and even be a permanent finding.
Fig. 7.19 Postablation appearance for RCC. (A) Baseline axial CT shows a 2.5-cm left renal mass (arrow). A larger mass (asterisk) is noted in the right kidney, and was planned to be treated with radical nephrectomy after ablation of the contralateral lesion. Three-month axial (B) and coronal (C) follow-up CT scans show the postablation rim completely surrounding the mass, a secondary sign of adequate ablation. Again the most important indication of a successful ablation is the lack of enhancement of the mass.
Preablation Embolization
Currently there are no guidelines regarding the preventive embolization of RCC prior to ablation. The decision for embolization is up to the surgeon and is purely dependent on the individual risk. Certainly there is no need to embolize smaller tumors (i.e., <5 cm) in relatively healthy individuals. On the other hand, embolization could prove lifesaving for an older patient with a large RCC who needs to restart anticoagulation soon after ablation. To place this in perspective, in our series of more than 100 RCC renal cryoablations we had 15 large hemorrhages (distorted anatomy and caused symptoms) that required no intervention. More importantly, we had two patients who required transfusions for low hematocrit. Interestingly, both patients restarted Coumadin anticoagulation 1 week postablation, and both patients presented with delayed retroperitoneal bleed soon after that. If clinically feasible, we currently delay anticoagulation for 2 weeks postablation. Unpublished data suggest preventive embolization may be useful for RCCs larger than 5 cm. The risk of hemorrhage is dependent on the number of probes (cryoablation) or probe insertions (thermal ablation), and is slightly less with the latter. One additional advantage offered by embolization is that the diminished blood flow may allow for more effective ablation (Fig. 7.24).
Fig. 7.20 (A) Axial contrast-enhanced (arterial phase) CT image 3 months postablation of a large left renal mass. Residual viable RCC is demonstrated by nodular enhancement at the margin of the ablated mass (arrow). (B) Axial contrast enhanced (venous phase) CT image 3 months postablation in a different patient shows very subtle thin linear enhancement at the medial margin of the RCC, suggestive of residual viable tumor (arrow). Meticulous examination of all phases of a contrast-enhanced follow-up CT or MRI is necessary, as some masses may be invisible in one phase.
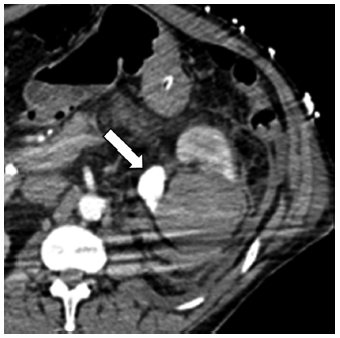
Fig. 7.21 Axial contrast-enhanced CT immediately postablation. The study was obtained for suspicion of clinically significant hemorrhage, which was not the case. However, the study revealed delayed and persistent enhancement of a segment adjacent to the ablated mass (arrow). This finding is not uncommon (see Fig. 7.11) and likely represents segmental acute tubular necrosis. No segmental acute tubular necrosis was noted on the follow-up 3-month CT, which suggests this finding may be more frequent than thought but one that is always self-limiting. No intervention is necessary.
- Compared with the baseline supine cross-sectional imaging, anatomy may change for better or worse once the patient is placed prone for renal ablation.
- Renal cryoablation does not require general anesthesia. Intraprocedurally, there is minimal to no pain; however, significant pain may develop during recovery as the ice ball thaws and infammation sets in.
- Most kidney ablation patients can be treated on an outpatient basis.
- The 3-month follow-up cross-sectional imaging may show a lesion that is stable in size despite adequate ablation; however, there should be no enhancement. As a rule, the 6-month study should show a lesion decreasing in size.