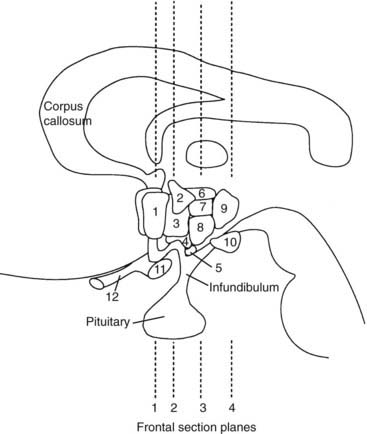
FIGURE 12-1. Schematic representation of lateral brain section demonstrating hypothalamic nuclei. Dashed lines represent the frontal (coronal) section planes illustrated in Figures 12-2 and 12-3. 1, preoptic nucleus; 2, paraventricular nucleus; 3, anterior hypothalamic areas; 4, supraoptic nucleus; 5, arcuate nucleus; 6, dorsal hypothalamic area; 7, dorsomedial nucleus; 8, ventromedial nucleus; 9, posterior hypothalamic area; 10, mamillary body; 11, optic chiasm; 12, optic nerve.
(From Braunstein GD: The hypothalamus. In Melmed S [ed]: The Pituitary, 2nd ed. Cambridge, MA: Blackwell Scientific, 2002, pp 317–348.)
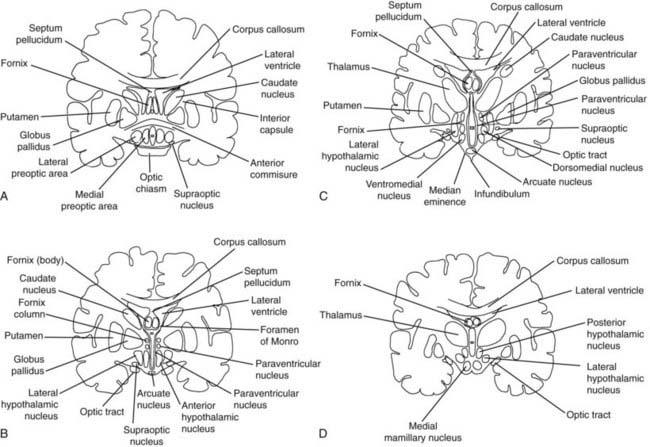
FIGURE 12-2. Frontal (coronal) sections of the hypothalamic regions. A, Preoptic region (frontal section plane 1 in Fig. 12-1). B, Supraoptic region (frontal section plane 2 in Fig. 12-1). C, Tuberal region (frontal section plane 3 in Fig. 12-1). D, Mamillary region (frontal section plane 4 in Fig. 12-1).
(From Braunstein GD: The hypothalamus. In Melmed S [ed]: The Pituitary, 2nd ed. Cambridge, MA: Blackwell Scientific, 2002, pp 317–348.)
The hypothalamus is responsible for many of the body’s homeostatic mechanisms, including water metabolism, temperature regulation, appetite control, the sleep/wake cycle, circadian rhythms, and control of the sympathetic and parasympathetic nervous systems. In addition, this area has activity in regard to emotional expression, behavior, and memory. Finally, the hypothalamus is essential to the neuroendocrine control of anterior pituitary function. Table 12-2 lists the various functions of the hypothalamus, the hypothalamic nuclei or hypothalamic regions that have been identified as being responsible for these functions, and the disorders that result from either destructive or stimulatory lesions in or around the nuclei or region.1,4–25
Table 12-2. Hypothalamic Functions, the Nuclei or Regions Involved with the Specific Functions, and the Disorders Resulting from Stimulatory or Destructive Lesions in the Regions
| Function | Nuclei [n] or Region Involved [r] | Disorders |
|---|---|---|
| Water metabolism | ||
| Temperature regulation | ||
| Appetite control | ||
| Sleep/wake cycle and circadian rhythm | ||
| Visceral (autonomic) fraction | ||
| Emotional expression and behavior | ||
| Memory | Short-term memory loss | |
| Control of anterior pituitary |
SIADH, Syndrome of inappropriate secretion of antidiuretic hormone.
Hypothalamic Disorders: Pathophysiologic Principles
First, the small overall size of the hypothalamus and the close association of the nuclei and nerve tracts mean that a variety of different pathologic processes may give rise to the same signs and symptoms of neurologic and hypothalamic dysfunction.4 The spectrum of disorders that can affect the hypothalamus is shown in Table 12-3. Tumors, infiltrative disorders, and infections, among other conditions, frequently give rise to headaches, neuro-ophthalmologic disorders, pyramidal tract or sensory nerve dysfunction, extrapyramidal cerebellar signs, and recurrent vomiting.9,10 Other common manifestations include gonadal dysfunction (either hypogonadism or precocious puberty), diabetes insipidus, somnolence, dysthermia, and evidence of a caloric imbalance (either with hyperphagia and obesity or anorexia with emaciation).9,10
Table 12-3. Causes of Hypothalamic Dysfunction
| Congenital |
| Tumors |
| Infiltrative |
| Immunologic |
| Nutritional, Metabolic |
| Degenerative |
| Infectious |
| Vascular |
| Trauma |
| Functional |
| Other |
DIDMOAD, Diabetes insipidus, diabetes mellitus, optic atrophy, deafness.
Modified from Braunstein GD: The hypothalamus. In Melmed S (ed): The Pituitary, 2nd ed. Cambridge, MA: Blackwell Scientific, 2002, pp 317–348.
Second, although exceptions exist, most patients who have a systemic disorder such as Langerhans’ cell histiocytosis, sarcoidosis, tuberculosis, or leukemia will exhibit manifestations of the disease outside the hypothalamus and central nervous system.
Third, a lesion may disrupt a function that is subserved by a hypothalamic nucleus distant from the lesion. Because the afferent and efferent tracts to and from the hypothalamic nuclei traverse other areas of the hypothalamus and brain distant from the nuclei, lesions that affect those tracts may result in dysfunction of several hypothalamic nuclei.
Fourth, most lesions that result in chronic hypothalamic syndromes involve more than one nucleus. As can be seen in Table 12-2, most of the hypothalamic functions are controlled by more than one nucleus, and this redundancy allows some degree of compensation should one nucleus be affected. In addition, most of the nuclei are paired, and destruction of a single nucleus may not be sufficient to result in a clinical syndrome. Thus lesions that affect the basal tuberal region of the hypothalamus (pituitary adenomas with suprasellar extension, optic gliomas, and craniopharyngiomas), are multiple (granulomatous disorders, metastatic tumors, infiltrative disease), or cause enlargement of the third ventricle (aqueductal stenosis, colloid cysts, pinealomas, germ cell tumors, midbrain gliomas) will more likely result in clinical hypothalamic dysfunction than will disorders affecting the more lateral portions of the hypothalamus.
Fifth, the rate of progression of the pathologic process affects the patient’s clinical manifestations. Slowly progressive lesions may give few or no symptoms until they achieve a large size, at which time, altered endocrine function and deterioration of cognitive ability may be present. Small, acute lesions may result in profound clinical manifestations such as alterations in consciousness, thermal dysregulation, and diabetes insipidus.
Sixth, the clinical syndrome due to involvement of a hypothalamic nucleus or tract may differ depending on whether the pathologic lesion is destructive or stimulatory. As an example, chronic, destructive lesions of the preoptic region may result in hypothermia and insomnia, whereas hyperthermia and lethargy may be seen with acute stimulatory lesions.
Finally, the clinical manifestations of the hypothalamic disease depend in part on the age of the patient. Thus prepubertal gonadotropin deficiency results in sexual infantilism, whereas in the postpubertal state, regression of secondary sexual characteristics (but not disappearance) occurs. Similarly, prepubertal growth hormone deficiency due to a hypothalamic lesion disturbing growth hormone–releasing hormone (GHRH) function results in short stature, whereas a similar lesion occurring in an adult may manifest only as adult growth hormone deficiency syndrome.
Manifestations of Hypothalamic Disease
DISORDERS OF WATER METABOLISM
Central Diabetes Insipidus
Complete or partial central diabetes insipidus results from (1) destruction of the antidiuretic hormone (ADH)-producing magnocellular neurons in the supraoptic and paraventricular nuclei or (2) interruption of the transport of ADH through their axons, which terminate in the pituitary stalk and posterior pituitary. Diabetes insipidus is relatively common in patients with chronic hypothalamic disorders, being found in approximately 35% of such patients.9,10 It also is frequently found in patients with acute insults to the hypothalamus or pituitary stalk, as is seen in vascular accidents and neurosurgical trauma. Obesity and hypogonadism frequently are present in patients with diabetes insipidus due to tumors or infiltrative disorders (Fig. 12-3).
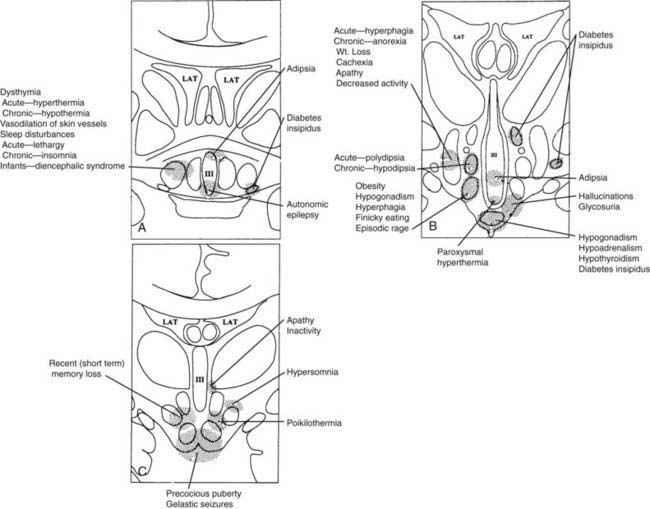
FIGURE 12-3. Clinical findings associated with hypothalamic lesions located at various anatomic sites. Clinicopathologic correlation based on multiple studies.15–25 A, Corresponds to region depicted in Figure 12-2A. B, Corresponds to region depicted in Figure 12-2C. C, Corresponds to section depicted in Figure 12-2D.
(From Braunstein GD: The hypothalamus. In Melmed S [ed]: The Pituitary, 2nd ed. Cambridge, MA: Blackwell Scientific, 2002, pp 317–348.)
The majority of patients with diabetes insipidus have idiopathic or familial diabetes insipidus associated with gliosis of the supraoptic and paraventricular nuclei.26 Approximately one third of patients with idiopathic diabetes insipidus have detectable anti-ADH–producing cell antibodies, suggesting an autoimmune cause.27 Autosomal-recessive, X-linked-recessive, and autosomal-dominant forms of familial diabetes insipidus have been described. In the more common autosomal-dominant form, nucleotide deletions or substitutions in the ADH gene on chromosome 20 have been identified.28 The DIDMOAD syndrome (Wolfram’s syndrome) represents a rare autosomal-recessive form of central diabetes insipidus (DI) associated with type 1 diabetes mellitus (DM), optic atrophy (OA), bilateral sensorineural deafness (D), and occasionally ataxia and autonomic neurogenic bladder.29 Diabetes insipidus is a frequent manifestation of suprasellar and pineal germinomas, sarcoidosis, lymphocytic infundibuloneurohypophysitis, and the chronic disseminated form of Langerhans’ cell histiocytosis.30–37
Adipsic or Essential Hypernatremia
Adipsic hypernatremia occurs when the osmoreceptors that are present in the anterior medial and anterior lateral preoptic regions are damaged. Affected patients have an impaired thirst mechanism, which results in insufficient fluid intake despite the hypernatremia. Although most of the affected patients have partial diabetes insipidus, their extracellular fluid volume remains normal, and they are not dehydrated. Therefore, they exhibit chronic elevations of serum sodium but normal blood pressure, pulse rate, serum creatinine, and creatinine clearance and can release ADH and concentrate their urine during fluid deprivation. When serum sodium concentrations are less than 160 mmol/L, few symptoms are present. However, between 160 and 180 mmol/L, patients may have fatigue, weakness, lethargy, muscle tenderness, cramps, anorexia, depression, and irritability; at 180 mmol/L, stupor and coma may be present. Close to half of these patients have hypothalamic obesity, and almost three fourths demonstrate some degree of anterior pituitary hormone deficiency.6,9,10,13,38,39
Essential hypernatremia has been described with a variety of lesions, including craniopharyngiomas, suprasellar germinomas, optic nerve gliomas, pineal tumors, Langerhans’ cell histiocytosis, sarcoidosis, trauma, hydrocephalus, cysts, inflammatory conditions, ruptured aneurysms of the anterior communicating artery, and toluene exposure.38–40 The Hayek-Peake syndrome is the association of essential hypernatremia with hypodipsia, obesity, lethargy, increased perspiration, central hypoventilation, hyperprolactinemia, hypothyroidism, and hyperlipidemia without an identifiable structural hypothalamic defect.41
Syndrome of Inappropriate Secretion of Antidiuretic Hormone
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is characterized by serum hyponatremia and hypo-osmolarity, with an inappropriately elevated urine osmolarity, in a patient with normal renal, adrenal, and thyroid function and no clinical evidence of intravascular or extracellular fluid volume expansion. The clinical symptoms depend on the rate of decrease of serum sodium, as well as the absolute serum sodium concentration. At serum sodium levels greater than 120 mmol/L, symptoms are generally mild and nonspecific and include anorexia, nausea, headache, weakness, and lethargy. At less than 120 mmol/L, these symptoms are accompanied by nausea, vomiting, and mental confusion; at very low levels, by seizure and coma.42 The syndrome is found with a variety of intracranial abnormalities, including head trauma, intracranial bleeding, meningitis, encephalitis, neurosurgery, hydrocephalus, acute intermittent porphyria, craniopharyngiomas, germinomas, and pinealomas.6,13,30 An idiopathic form has been described in young women who exhibit menstrual irregularities, have enlarged lateral ventricles, and have SIADH cyclically. No structural defect has been described in these patients.4
Reset Osmostat
The osmoreceptors, located in the circumventricular organs of the lamina terminalis, may become “reset” in some patients with tuberculosis, malnutrition, quadriplegia, and psychosis. The osmoreceptors activate release of ADH at a lower serum osmolality than normal and appropriately decrease ADH release when the serum osmolality falls further.42
Cerebral Salt Wasting
Hyponatremia and the other manifestations of SIADH also co-occur in the syndrome of cerebral salt wasting, which is primarily seen in postoperative neurosurgical patients treated for subarachnoid bleeding, intracranial aneurysms, or following head injury. In contrast to SIADH in which patients are euvolemic or have an expansion of their effective arterial blood volume, patients with cerebral salt wasting are hypovolemic due to renal salt loss. The salt wasting and hypovolemia are felt to be the result of disruption of the normal sympathetic nervous system input into the kidneys and/or relapse of a natriuretic factor from the brain.43
DYSTHERMIA
Hyperthermia
The warm receptors present in the preoptic anterior hypothalamus are stimulated by an increase in the temperature of the blood. Together with signals from peripheral warm receptors that respond to an increase in external temperature, the afferent signals travel through the median forebrain bundle to the lateral portion of the posterior hypothalamus, which leads to vasodilation and sweating to dissipate heat. Conversely, stimulation of the preoptic anterior hypothalamic cold receptors through a decrease in temperature of the blood, or stimulation of the peripheral cold receptors through a decrease in ambient temperature, results in medial neurons in the posterior hypothalamus activating heat production through muscular shivering and heat conservation through vasoconstriction.5,6
Acute injury to the anterior hypothalamic and preoptic areas may result in a rapid temperature elevation (as high as 41°C) associated with tachycardia and unconsciousness from failure of the heat-dissipating mechanisms to function while heat production continues. Chronic hyperthermia may be secondary to lesions in the tuberoinfundibular region. In contrast to patients with elevated temperature due to the inflammation of infections, these patients generally do not experience malaise and paradoxically may have peripheral vasoconstriction.1,5,6,9,11
Wolff and colleagues44 described a syndrome of hyperthermia associated with shaking chills, fever, hypertension, vomiting, and peripheral vasoconstriction that occurred cyclically at 3-week intervals, without a pathologic lesion in the hypothalamus being found. Similar paroxysms of hyperthermia have been noted in other patients without the cyclicity, and together these episodes may represent a variant of diencephalic epilepsy.6,44,45
Between 0.02% and 2.4% of patients receiving neuroleptic drugs develop the neuroleptic malignant syndrome (NMS), which is characterized by hyperthermia to 38°C or higher; severe extrapyramidal signs, including “lead-pipe” muscle rigidity and tremor; signs of autonomic nervous system dysfunction such as pallor, tachycardia, arrhythmias, blood pressure lability, and diaphoresis; and changes in mental status, including mutism, delirium, and coma.46 All antipsychotic medications have been reported to cause NMS, and most evidence suggests that disruption of dopamine neurotransmission by neuroleptic-induced dopamine receptor blockade is the major pathophysiologic abnormality in susceptible individuals. Indeed, the greater the potency of the neuroleptic in regard to its dopamine D2-receptor antagonism activity, the greater the frequency of NMS occurrence.46 NMS is successfully treated with a variety of dopamine agonists. Injury to the preoptic medial and tuberal nuclei has been demonstrated at autopsy, as has a depletion of hypothalamic norepinephrine concentrations.47 The syndrome generally begins within 2 weeks of initiating the neuroleptic and evolves over a 24- to 72-hour period. The most common complication is rhabdomyolysis, which may result in myoglobinuria and acute renal failure. The mortality of this syndrome is currently less than 10%, which reflects increased recognition of the disorder and initiation of prompt therapy.46
The serotonin syndrome is closely related to NMS and presents with the triad of altered mental status (somnolence, confusion, agitation, seizures, and coma), autonomic instability (fever, diaphoresis, tachycardia, hypo- or hyperthermia, mydriasis), and abnormal neuromuscular activity (myoclonus, rigidity, hyperreflexia). Any drug or combination of drugs which elevates the concentration of serotonin in the central nervous system can cause the syndrome. These include selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, monamine oxidase inhibitors, cocaine, and amphetamines. These drugs either directly or indirectly activate thermogenesis via the hypothalamus.46
Hypothermia
Large, destructive lesions of the anterior or posterior hypothalamus may result in inability to generate heat through vasoconstriction and muscular shivering. This occurs in 10% to 15% of patients with a variety of hypothalamic lesions, especially neoplasms, infiltrative disorders, and infections.9,10,13 It also has been noted in patients with Parkinson’s disease and Wernicke’s encephalopathy, which are associated with lesions in the posterior hypothalamus and mamillary bodies, respectively.48,49
Diencephalic autonomic epilepsy refers to episodic or paroxysmal hypothermia during which the body temperature decreases to 32°C or less over minutes to days, along with evidence of autonomic nervous system dysfunction, including flushing, sweating, hypotension, bradycardia, salivation, lacrimation, pupillary dilation, Cheyne-Stokes respiration, nausea, vomiting, asterixis, ataxia, and obtundation.6,15,50–53 Electroencephalographic (EEG) abnormalities occur during the episodes. Autopsy studies have shown gliosis and loss of the arcuate nucleus and premamillary area in some patients, whereas others have been found to have tumors involving the floor and lower portion of the third ventricle.15,50 The corpus callosum has been found to be absent in approximately half of patients with episodic hypothermia; these individuals may also exhibit diabetes insipidus, reset osmostat, growth hormone deficiency, hypogonadism, or precocious puberty (Shapiro’s syndrome).54
Poikilothermia
When both the heat-loss and heat-conserving homeostatic mechanisms are impaired, wide fluctuations of body temperature might take place without the patient’s experiencing thermal discomfort. This condition, known as poikilothermia, is found with both anterior and posterior hypothalamic destruction, as well as in patients with large lesions that may involve the posterior hypothalamus and rostral mesencephalon.6,9,10 Rarely, patients with Wernicke’s encephalopathy may experience poikilothermia.6
DISORDERS OF APPETITE CONTROL AND CALORIC BALANCE
Hypothalamic Obesity
Approximately 25% of patients with structural hypothalamic lesions exhibit hyperphagia and obesity.9,10 Usually, the patients have lesions involving a large portion of the hypothalamus, although bilateral destruction of only the ventromedial nucleus may lead to hypothalamic obesity.1,9,10,17,18,22,25,55 The majority of patients harbor a neoplasm, especially craniopharyngioma, with a minority having inflammatory or granulomatous processes, a history of trauma, or infiltrative disorders.55 Common clinical findings in these patients include headaches, visual abnormalities, hypogonadism, diabetes insipidus, and somnolence. Less commonly, behavioral abnormalities, such as antisocial behavior or sham rage, and seizures may be present.55 Hypothalamic obesity also is found with defects of the leptin and leptin receptor genes, the melanocortin 4 receptor gene, and the proopiomelanocortin gene.56–59
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree