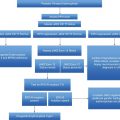
Pedigree of family with myeloproliferative neoplasm. Filled symbols indicate affected individuals. The Roman numerals represent generation and the Greek numeral stands for the individual family member. PV, polycythemia vera; MM, multiple myeloma; ET, essential thrombocythemia.
While PV in multiple family members has been described in multiple reports over many decades, many of these reports lacked the rigorous differentiation of PV from inherited polycythemic states by erythropoietin-independent erythroid colonies (EEC) assay.8 In point of fact, in our experience, several patients with EPOR and HIF2α mutations were erroneously misdiagnosed as PV, even at prestigious referring medical institutions. Those errors are facilitated by the fact that some of these congenital disorders also have erythryopoietin-hypersensitive erythroid progenitors.9 The discovery of a specific PV molecular defect (JAK2 V617F)10 greatly facilitated accuracy of the PV diagnosis.
However, familial MPN clustering was described even before the specific PV molecular defect (JAK2 V617F) was defined. Our group described six families wherein EEC confirmed the PV phenotype in clonogenic cultures in the absence of exogenous erythropoietin3 and, in all instances, each individual was rigorously proven to have had previously normal hematocrits on repeated occasions. We thus determined that, in all affected individuals, the phenotype was clearly acquired. Further, in one family, a female relative with normal blood counts and circulating polyclonal platelets and granulocytes had EEC; we determined that these EEC utilized the same X-chromosome allele, indicating the presence of an as-yet asymptomatic PV clone. In contrast, her female relatives with a PV phenotype had clonal platelets and granulocytes and, when tested, reticulocytes. This suggests that, at least in this subject, the presence of a clone with limited contribution to total blood production constituted a pre-phenotype PV. Prior to the discovery of the JAK2 V617F mutation, we found that about one-half of PV patients in these six reported families had uniparental disomy (UPD) on chromosome 9p, the location of JAK2 V617F. However, in these families, there was no linkage between the PV phenotype and UPD on chromosome 9p (or chromosomes 20q, 13q, and 5q, locations of other reported recurrent cytogenetic MPN abnormalities).3,11 This indicates that these genetic events are not invariable predisposing germline determinants of MPNs. In one family, PV was acquired at age 5 in the propositus and in the third decade of life in his father, while the age of onset of PV in the remaining families was comparable to sporadic PV. Further, in one of our as-yet unpublished families, two of nine siblings had JAK2 V617F-positive ET, and another sibling with normal blood counts had a low, repeatedly detectable JAK2 V617F allelic burden (~1%) that disappeared in subsequent follow-ups. This suggests that, in some instances, the MPN-initiating clone may be very small and may have a limited lifespan.
JAK2 V617F mutation in familial MPN
A large French study extended our understanding of familial MPN clustering and examined the role of the JAK2 mutation in phenotype development in familial MPNs. In this report, at least two MPN cases were present in each family. This study included a total of 72 families with 174 affected MPN patients. Of these patients, 81 had PV, 68 had ET, 11 had primary myelofibrosis (PMF), one had systemic mastocytosis, and one had chronic myelomonocytic leukemia (CMML).4 Among the 72 families, 54 families had two affected members, 12 had three affected members, and six had more than three affected members. In some instances, one or more types of MPNs were observed within these families, e.g., PV, ET, or PMF or, in some families, other phenotypically related myeloid malignancies, including chronic myeloid leukemia (CML) or systemic mastocytosis. Within these familial clusters, 67% of the families had a homogeneous pattern of clinical phenotypes, i.e., characterized by family members with the same subtype, the majority being PV–PV pairs (50%) and ET–ET pairs (35%). However, in approximately 33% of families, a mixed phenotype was observed, with some affected relatives having PV, while others have ET and/or other MPNs. In 36 of 72 families, affected individuals were present in two generations, while in four families the affected individuals were present in three generations. In another 32 families, all affected individuals were present in a single generation and, thus, inheritance could not be determined.
Analyses of these pedigrees indicated that the distribution of MPN phenotypes within families was most compatible with autosomal-dominant inheritance with incomplete penetrance, supporting our previous observations.3 The JAK2 V617F mutation was found in approximately 75% of patients with PV and PMF and in 50% of patients with ET; the JAK2 V617F mutation was not detected in the other types of MPNs, similar to JAK2 V617F-positivity in sporadic MPNs. Analysis of the relationship of the JAK2 V617F mutation with hematologic and clinical profiles showed that JAK2 V617F-mutated patients had more thrombotic events, myelofibrosis, and leukemic transformation. These complications were also more frequently associated with a high allelic burden of the JAK2 V617F mutation. EEC were found in 9.6% of the healthy relatives without any MPN phenotype, and three of these individuals were positive for the JAK2 V617F mutation in EEC and/or blood mononuclear cells. In one of these previously healthy subjects, transformation to the PV phenotype was observed (increase in hematocrit from 45% to 54%). To examine whether the JAK2 V617F mutation is responsible for familial clustering of MPNs, the authors analyzed JAK2 V617F-positive blood mononuclear cells and their purified subpopulations, i.e. CD3+T cells, CD19+ B cells, and CD56+natural killer (NK) cells, from 22 families. In 13 unrelated myeloproliferative disorder patients, the JAK2 V617F mutation was absent in purified blood cell populations (CD3+ T cells, CD19+ B cells), further confirming that the JAK2 V617F mutation is acquired in familial MPNs and represents a secondary genetic event. Moreover, genome-wide linkage analysis also excluded a dominantly inherited MPN-predisposing locus in chromosome 9 (where JAK2 is located), supporting our previously published data.3 However, the JAK2 V617F mutation was present in 68% of MPN patients in NK+ cells and correlated with higher mutant allelic burdens in blood mononuclear cells. These data are congruent with the JAK2 V617F mutation originating in a multipotent hematopoietic progenitor cell.
In the same year as the French study, 485 MPN Italian sporadic cases were reported, with a prevalence of familial cases of 7.6% (35 pedigrees; 75 patients).5 The clinical phenotypes of the patients with familial MPNs were compared with patients with sporadic MPNs, and did not show significant differences. The distribution of clinical phenotypes within the same families showed similar patterns to the French study,4 with 60% of families having all affected members represented by the same phenotype and 40% of families having relatives with different MPN phenotypes. The presence of the JAK2 V617F mutation among patients with familial and sporadic MPNs showed a similar distribution. Interestingly, age distribution between patients in first and second generations showed a higher age-dependent hazard of MPNs, as well as a younger age at diagnosis, in the second generation. These observations are suggestive of disease anticipation in MPN.
The most persuasive evidence of the quantitative prevalence of germline predisposition of MPNs was provided by the Swedish Registry data, the largest population-based case-control study which included more than 11,000 MPN patients and their almost 25,000 linkable first-degree relatives, and almost 100,000 controls.6 A five- to seven-fold elevated risk of developing MPNs was shown among first-degree relatives of any MPN patients, excluding ET, which showed a 12-fold risk of ET in first-degree relatives of ET patients. However, in this study, the age at diagnosis between parents versus offspring showed no significant difference, failing to support disease anticipation, as reported by the Italian study.5 The risk of the other complications such as CML, acute myeloid leukemia, and myelodysplastic syndrome was analyzed, and only an increased risk of CML was observed in first-degree relatives. Moreover, when other hematological malignances and solid tumors were taken into consideration, evidence of a higher risk of chronic lymphocytic leukemia, malignant melanoma, and brain cancer was observed. The molecular basis of these observations still remains to be defined.
Role of X-chromosomes in development of MPN in females
When the risk of developing MPNs was analyzed by the sex of the affected individuals and their relatives, a higher risk was found in females than in males.6 This may indicate that some sex-specific biologic mechanism(s) may be involved in modulation of MPN phenotypes. This gender discrepancy may be related to a report that Xist, a gene encoding long-non-coding RNA initiating X-chromosome inactivation, when deleted in hematopoietic stem cells in female mice, leads to reactivation of inactive X-chromosomes.12 Importantly, all these female mice with reactivation of inactive X-chromosomes developed a highly aggressive MPN-like disease that progressed to invariably fatal lymphoma or acute leukemia.12 This study raises the possibility that reactivation of some regions of the X-chromosome in MPN clones in females may contribute to the gender diversity of PV and ET phenotypes observed in some clinical studies.13 In point of fact, we have observed that, in some females, there is regional hypomethylation of some X-chromosome regions, with some PV and ET individuals having both X-chromosome alleles of certain genes expressed in their PV/ET clone.
Somatic mutations in familial MPN pathogenesis
The acquired JAK2 V617F mutation present in most PV and almost half of ET and PMF sporadic or familial cases of MPNs does not explain the phenotypic variability. Additional genetic events, either germline or acquired, are thus likely involved in the development of MPN phenotypes. Genome-wide association studies of sporadic MPNs revealed many other somatic mutations in addition to the activating mutation of JAK2. These recurrently observed additional mutations include cMPL, CALR, ASXL1, EZH2, SH2B3, DNMT3A, and inactivating mutations of putative tumor suppressor gene TET2.14 However, JAK2 V617F and MPLW515K/L have never been found in germlines, but other germline mutations of JAK2 or cMPL genes (JAK2 V617I, cMPLS505N) have been found in rare sporadic families with hereditary thrombocytosis. Further, a germline mutation of TET2 was described in two sisters, one with JAK2 V617F-positive PV, the other without an MPN phenotype.2 This suggests that, in this family, the TET2 mutation was a facilitating, but not essential, factor in PV clonal development. However, in another study of 42 MPN families, 12 members harbored various distinct TET2 mutations, and analysis of familial segregation confirmed that the TET2 mutations were not inherited but somatically acquired.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree