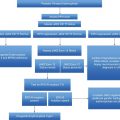
Local management protocol. FBC, full blood count; BP, blood pressure; PV, polycythemia vera.
There are no controlled studies regarding pregnancy outcome. As such there are no validated predictors of pregnancy outcome. However, previous thrombosis, hemorrhage, and extreme thrombocytosis in women with ET are considered risk factors for fetal/maternal complications.8 Previous pregnancy outcome, age, thrombophilia, platelet count, leukocyte count, and hemoglobin level have not been demonstrated to be predictive of pregnancy outcome in ET.5,9,10 Whether or not the JAK2 V617F mutation is a predictor of poor pregnancy outcome is not as yet clear and as such it has not been incorporated into the local pregnancy risk assessment. Passamonti et al. suggest the presence of the JAK2 V617F mutation is a predictor of poor pregnancy outcome and that aspirin may be effective in the prevention of pregnancy complications in JAK2 V617F-positive women with ET.10,11 This association of the JAK2 V617F mutation and poor pregnancy outcome is supported by Melillo et al.5; however, aspirin did not alter the effects of the JAK2 V617F mutation. In general, interferon appeared to improve outcome (p = 0.034, odds ratio 0.1, 95% confidence interval 0.013–0.846), although any potential capability to overcome the unfavorable effect of the JAK2 V617F mutation remains undefined. In contrast, Gangat et al.7 report that aspirin may be associated with a favorable outcome, but there is no association between the presence of the JAK2 V617F mutation and pregnancy loss.
Case 1 would be considered standard risk in the absence of any additional risk factors and advice would be to continue aspirin throughout pregnancy with the addition of low-molecular-weight heparin thromboprophylaxis for 6 weeks postpartum. Aspirin has been shown to be safe in low doses by the Collaborative Low-dose Aspirin Study in Pregnancy (CLASP).12 The woman would be followed up monthly in hematology to include a full blood count review, blood pressure monitoring, and urinalysis. In addition to midwifery care the patient would receive obstetric review. In low-risk MPN pregnancy local practice does not support commencing cytoreductive therapy to reduce platelet count unless it is greater than 1500 × 109/L. This is unlikely in this case, as the increased plasma volume in pregnancy is likely to result in a lower platelet count. In addition to the anomaly scan at 20 weeks, uterine artery Dopplers would be performed and guide subsequent frequency of growth scans. Local practice is to support breastfeeding whilst maintaining low-dose aspirin. Aspirin is excreted into breast milk at low concentrations. In view of immature metabolism and accumulation in the infant’s serum, this may theoretically result in platelet dysfunction and Reye’s syndrome. However, there is only one report of toxicity in a breastfed infant despite extensive use and the dose used in MPN is low.
Postpartum follow-up within 6 weeks allows review of any complications and blood parameters, which rapidly return to prepregnancy levels.
The management of this case outlines a standard-risk protocol for MPN in pregnancy. In PV the only addition to a standard-risk protocol would be venesection. For the subsequent cases this standard of care would also apply and further cases only detail additional management.
Case 2: part 1
A woman attends clinic for prepregnancy counseling. She is 32 years old and has a history of an estrogen-provoked thrombosis at the age of 27 whilst taking the oral contraceptive pill and three subsequent first-trimester pregnancy losses. In view of the previous estrogen-provoked event in all 3 pregnancies she received low-molecular-weight heparin thromboprophylaxis. A diagnosis of ET was made following referral to a hematologist after review of historical platelet counts in the 3 pregnancies, as these were abnormally high. At this time her platelet count was 1621 × 109/L. Following a diagnosis of JAK2, MPL-negative ET she was commenced upon 75 mg aspirin following exclusion of an acquired von Willebrand’s and pegylated interferon. Current platelet count is stable at 348 × 109/L with a dose of pegylated interferon of 135 μg subcutaneously every 3 weeks.
In the absence of review of full blood counts at the time of the thrombosis it is not possible to determine whether the diagnosis of ET was present at this time. Regardless, this woman would be offered heparin thromboprophylaxis in pregnancy continued until 6 weeks postpartum in view of the previous estrogen-provoked event. The three first-trimester losses alone would suggest this pregnancy is at increased risk and as such in addition to standard-risk protocol management and heparin it would be advisable to continue the interferon-alpha.
Low-molecular-weight heparin is safe in pregnancy according to an extensive systematic review.13 Neither unfractionated nor low-molecular-weight heparin crosses the placenta, and there is no evidence of teratogenesis or fetal hemorrhage. In women with a history of pregnancy morbidity the local increased risk protocol is to offer a weight-adjusted prophylactic dose of low-molecular-weight heparin once daily throughout pregnancy. In women with a previous thrombosis, as in this case, the same weight-adjusted prophylactic dose of low-molecular-weight heparin is commenced and increased to twice daily from 16 weeks. Heparins are not excreted in breast milk and may be used safely when breastfeeding.
No cytoreductive drugs discussed in this chapter have a license for use in pregnancy. Several reports have suggested the safety of interferon-alpha in pregnancy, with no effect on the fetus, and there are no reports of teratogenicity in animals. However, there is some evidence to suggest interferon-alpha decreases fertility and is best avoided in women with difficulty conceiving.14 Recommendation to avoid breastfeeding with interferon-alpha is based on reports that it is variably excreted in breast milk and may be active orally. There is an absence of safety data rather than evidence of harm to the neonate. Local practice is to discuss the theoretical risks alongside the benefits of breastfeeding and support the mother’s decision.
Case 3: part 1
A pregnant 26-year-old woman diagnosed with PV following investigation of menorrhagia and epistaxis 4 years previously is referred for an opinion at 9 weeks’ gestation. There is no prior obstetric history and the current management is aspirin 75 mg once daily and venesection. Bleeding symptoms resolved following commencing venesection. Hemoglobin is 145 g/L, hematocrit 0.47, platelets 499 ×109/L.
Although diagnosis followed investigation of menorrhagia and epistaxis, these are fairly soft indicators of risk and, with the knowledge that commencing regular venesection led to cessation of bleeding symptoms, this pregnancy could be managed as a standard-risk PV in pregnancy. In addition to standard-risk protocol the hematocrit should be maintained below 0.45 and maintenance of a gestational-appropriate target range considered (Table 17.2). Plasma expansion may obviate the need to venesect in pregnancy. If venesection fails to maintain an appropriate hematocrit or is not tolerated, cytoreductive therapy with interferon-alpha may be considered.
| First trimester | Second trimester | Third trimester | |
|---|---|---|---|
| Hemoglobin (g/L) | 110–143 | 100–137 | 980–137 |
| Hematocrit (L/L) | 0.31–0.41 | 0.30–0.38 | 0.28–0.39 |
| Platelet count × 109/L | 174–391 | 171–409 | 155–429 |
Case 4: part 1
A 36-year-old woman attends clinic. Obstetric history includes 2 pregnancies from a previous partner over 10 years ago, followed by a more recent pregnancy in the last year that sadly resulted in a stillbirth. The fetus was growth-restricted and the placenta was noted to be gritty with areas of infarction. During her bereavement appointment with the obstetrician to discuss her pregnancy, abnormalities in her sequential full blood counts were noted. The hematology registrar was contacted and it was suggested a JAK2 be sent and the woman be followed up in hematology. She attends clinic today and explains that she and her husband were very keen to try again, and she is already 7 weeks’ pregnant. Her JAK2 result is positive and full blood count reveals hemoglobin 157 g/L, hematocrit 0.49, and platelets 567 × 109/L.
This case serves as a reminder that full blood count parameters outside the appropriate gestational range should be investigated and, in the absence of an alternative cause, a diagnosis of MPN excluded. This woman should be counseled regarding the new diagnosis of PV. In view of the previous stillbirth with typical findings, including intrauterine growth restriction and placental infarction, the current pregnancy would be considered at increased risk. In addition to the standard-risk protocol this woman should be offered a once-daily weight-adjusted prophylactic dose of low-molecular-weight heparin to be continued until 6 weeks postpartum and interferon-alpha. It would be reasonable to perform a venesection in view of the current hematocrit and from here on titrate the interferon-alpha, aiming for a hematocrit and platelet count in the gestation-appropriate range. Local practice would include induction of labor if this woman had not delivered by her due date in view of the history of stillbirth.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree