FIGURE 30-1. Extreme cachexia in an anorexia nervosa patient.
(From Bliss EL, Branch CHH: Anorexia Nervosa: Its History, Psychology, and Biology. New York: Paul B. Hoeber, 1960.)
A corollary of this is the third criterion, disturbance of the experience of one’s body weight or shape (e.g., undue influence of body weight or shape on self-evaluation/self-esteem and/or denial of the seriousness of the weight loss), even though there may be clear adverse physical sequelae in addition to the current fourth criterion, secondary amenorrhea. For the endocrinologist, questions that should be asked of the patient and her family to address these psychological and behavioral aspects of AN include the following (adapted from the Structured Clinical Interview for DSM-IV37):
Rather than rigidly adhering to the DSM-IV-TR algorithm for diagnosing AN—that is, the reduction of body weight to less than 85% of expected and complete amenorrhea for at least 3 months—the clinician should consider both the patient’s core symptoms and current medical impairment; such impairment may require treatment even though body weight may be greater than 85% of expected and/or menses still may occur sporadically.31
For BN, the distinguishing features are uncontrollable binge eating of a definitely larger-than-normal amount of food (first criterion) that is clearly repetitive (third criterion). Here too, one’s sense of self is unduly influenced by weight and body shape (fourth criterion). Compensatory behaviors such as self-induced vomiting, purging, fasting between binges, and exercise are invoked to prevent weight gain (second criterion). The frequency and chronicity of the bingeing, and especially the compensatory behaviors, help distinguish BN from overeating in general. And because binge eating and purging can occur as part of AN as a subtype, a fifth criterion for BN is that it does not occur exclusively during episodes of AN. The validity of the DSM-IV-TR eating disorders classification has been questioned, owing to the high rates of diagnostic crossover among the AN subtypes and BN. Recent findings support the longitudinal distinction between AN and BN, but they may not support the AN subtyping schema.38,39 BED also is being distinguished from BN18,40 because obesity rather than inanition often results, and the endocrinologic and other metabolic changes are different from those of AN and BN.
For the endocrinologist, questions that should be asked of the patient and her family to address the criteria for BN include the following (adapted from the Structured Clinical Interview for DSM-IV37):
These questions and those regarding AN should be phrased in a way that is comfortable to both the health care professional and the patient. Depending on the patient, questions about both bulimic and anorexic behaviors may need to be asked; the patient’s history and presenting symptoms should dictate the emphasis of the interview.41 The sample questions are given to indicate that one can (and should) ask about eating behavior forthrightly. If the topics of the questions are followed, information about all the diagnostic criteria for both AN and BN will be gleaned. The patient should also be asked if this is the first time these behaviors have occurred or if there have been past episodes; in the case of the latter, a careful history taking about each episode, its severity and duration, and treatments and their success should be done. This information may yield important clues as to how to approach the patient therapeutically during the current episode.
Comorbid symptoms of depression and anxiety occur with some frequency in both AN and BN and should be assessed, because their persistence during treatment may portend poorer outcomes.42–48 Symptoms of depression and anxiety are exaggerated by malnutrition and improved with nutrition. AN patients with obsessive-compulsive disorder tend to have an obsessional need for symmetry/exactness and compulsions toward ordering and arranging.49 Mild to moderate negative mood states and obsessive symptoms can persist after recovery in both anorexic and bulimic individuals,50,51 suggesting that these traits may contribute to the pathogenesis of eating disorders.52 As well, comorbid impulse control disorders (ICD), the most common being compulsive buying disorder and kleptomania, occur more frequently in individuals with binge eating subtypes and are associated with greater use of laxatives, diuretics, appetite suppressants, and fasting.53
There also is a high incidence of comorbid obsessive-compulsive disorder (OCD) in anorexic and bulimic women and their families, as well as increased rates of AN and BN in persons with primary OCD. It may be that the core eating disorder symptoms (e.g., fear of fatness, pursuit of thinness) are a specific type of obsession. Symmetry, ordering, and perfectionism are the most common target symptoms in women with AN and BN and often persist after recovery. Leckman and others54 delineated four symptom dimensions of OCD, one of which was symmetry and ordering; these occurred most commonly in men. OCD in men and eating disorders in women may be gender-specific expressions of a common psychobiology related to obsessions with symmetry and ordering compulsions.
There are primary central nervous system (CNS) conditions for which anorexia or bulimia may be an associated symptom; these include both organic pathology55 and “functional” illnesses. As an example of the latter, one of the authors was asked to consult on a hospitalized adolescent girl with electrolyte disturbances suggestive of Bartter’s syndrome. With no evidence of somatic causes of the patient’s cachexia, and with nurses’ observations of bizarre eating habits, a diagnosis of AN was entertained, and a psychiatric interview of the patient during endocrine rounds was requested. After introductions as to who the psychiatrist was and why he was there, questioning began with what the patient ate (lettuce and carrots only) and why she ate only those two items (the computer in the hospital basement was giving her instructions to do so). Elucidation of additional symptoms of psychosis led to a presumptive diagnosis of schizophrenia with secondary anorexia and referral for inpatient psychiatric care.
Genetics
Eating disorders in both women and men are familial, as shown by both family and twin studies.56–61 The heritability of both AN and BN has been estimated between 33% and 84%.62 Recent findings corroborate previous research implicating the transition from early to mid-adolescence as a critical time for the emergence of a genetic diathesis for disordered eating.63 Studies of the genetics of AN and BN have been primarily by linkage analysis and association studies. In linkage analysis, correlations are determined between the occurrence of a disease in families and the inheritance of specific chromosomal regions in those families. In association studies, nucleotide polymorphisms are searched for in specific candidate genes suggested by chromosomal linkage studies and/or the known functions of the gene product.
Linkage studies in AN and BN families have suggested susceptibility genes on several chromosomes, but the strength of the associations has depended on the diagnostic stringency of the cases. For example, in 192 families with at least one affected relative pair with AN, BN, and related eating disorders, modest linkage was found with a marker on chromosome 4.64 However, when the analysis was restricted to 37 families in which at least two relatives had a diagnosis of the restricting subtype of AN (without bingeing and purging), there was a much stronger linkage to a marker on chromosome 1p. With reference to specific behavioral traits, two variables were identified in a sample of 196 families with an AN proband: drive for thinness and obsessionality.65 When these variables were incorporated into a linkage analysis, there again was highly significant linkage to a region on chromosome 1, as well as linkages of lesser significance to regions on chromosomes 2 and 13. In contrast, linkage analysis of 308 families with a BN proband yielded a high linkage score to chromosome 10.66 In a subset of 133 families in which two or more BN members reported self-induced vomiting, an even higher linkage was found to a region on chromosome 10p. Another region on chromosome 14q was suggestive of linkage. These studies can serve to suggest specific chromosomal regions for association studies.
In association studies, researchers have targeted serotonin, dopamine, and norepinephrine-related genes. The most promising results have been with the serotonergic system, because serotonin (5-HT) neurotransmission inhibits feeding behavior.56,67 Several studies have suggested an association between polymorphisms in the promoter region of the 5-HT2A receptor gene and both AN and BN, but the results have been inconsistent, the association has been rather weak, and the functionality of the 5-HT2A polymorphism is unknown.68,69 Studies reporting an association between a polymorphism in the promoter region of the 5-HT transporter gene and eating disorders have been inconsistent as well. In one study, a relationship was suggested between a polymorphism in the 5-HT1B receptor gene and BN; in another study, a relationship was suggested between a polymorphism in the 5-HT2C receptor gene and vulnerability to rapid weight loss following reduced food intake in AN. On the other hand, studies of the 5-HT receptor genes 2C, 7, and 1Dβ, as well as the tryptophan hydroxylase gene (the rate-limiting enzyme in the biosynthesis of 5-HT), have been negative. Phenotypically, reduced serotonergic activity, as measured by platelet 3H-paroxetine binding, has been found in both AN and BN and was unrelated to diagnostic subtype or ancillary dimensions such as impulsive behavior or depression.70 The use of serotonin uptake inhibiting antidepressants in the treatment of AN and BN will be discussed later.
The dopaminergic system also has received attention because dopamine (DA) neurotransmission has been implicated in feeding behavior and in motor activity, amenorrhea, and distortion of body image.56,57 One study each of D3 and D4 receptor polymorphisms and food restriction in AN have been negative. One of three studies of polymorphisms in the catechol-O-methyl transferase gene (an enzyme in DA metabolism) in AN was positive, but the other two were negative.
Other candidate genes controlling hormones and proteins putatively related to food intake that have been studied in eating disorders include the estrogen receptor, the uncoupling proteins UCP-2 and UCP-3, pro-opiomelanocortin, the melanocortin-4 receptor, leptin, agouti-related protein, neuropeptide Y, cholecystokinin (CCK), the β3-adrenergic receptor, and tumor necrosis factor. All but one have been negative.56,71 Thus, although the familial clustering of both AN and BN is clear, there is no solid evidence that single nucleotide polymorphisms in any of the aforementioned genes are other than mildly related to either of these eating disorders.
General Physical and Laboratory Findings
The general physical and laboratory findings of AN and BN are presented in Tables 30-3 and 30-4.72,73 Metabolic changes and medical complications occur secondary to chronic starvation and malnutrition and to bingeing and purging.7,73–75 Malnutrition-associated disturbances as severe as pulmonary bronchiectasis and emphysema have been reported.76 Cardiac complications are also well recognized in patients with eating disorders, including the more common abnormalities of bradycardia, orthostatic hypotension, and (although less common) the risk for more serious abnormalities, including prolongation of the QT interval and silent pericardial effusion.77
Table 30-3. Physical and Laboratory Findings in Anorexia Nervosa
Decreased plasma transferrin, complement, fibrinogen, prealbumin; usually normal protein and albumin:globulin ratio |
Adapted from Comerci GD: Medical complications of anorexia nervosa and bulimia nervosa. Med Clin North Am 74:1293–1310, 1990; and Mitchell JE, Crow S: Medical complications in adolescents with anorexia nervosa: a review of the literature. Curr Opin Psychiatry 19:438–443, 2006.
Table 30-4. Physical and Laboratory Findings in Bulimia Nervosa
Adapted from Comerci GD: Medical complications of anorexia nervosa and bulimia nervosa. Med Clin North Am 74:1293–1310, 1990.
Gastrointestinal (GI) disturbances occur in both AN and BN.78 Delayed gastric emptying and delayed colonic transit time resulting in constipation are common in AN patients and are associated with complaints of early satiety, bloating, and abdominal distension, leading the patient to feel fat and avoid eating. GI disturbances in BN include increased gastric capacity, decreased gastric relaxation, delayed gastric emptying, and abnormal function of the enteric autonomic nervous system. Abnormalities of GI function in AN and BN are reversible to various degrees with improvement in the underlying disorders. Treatments targeted at these abnormalities, such as agents to improve gastric emptying in AN and neurotransmitter modulators to reduce bingeing in BN, have met with varying success and require further study.
Increasing recognition is being given to osteopenia as an early and serious complication of AN.79–82 Adolescence is a time when optimal bone mineralization supporting physical growth is critical, and adolescent girls with AN have relatively poor bone mineral accrual. Several factors contribute to osteopenia in AN, including estrogen, androgen, and insulin-like growth factor deficiency; elevated cortisol, ghrelin, and peptide YY; excessive exercise; and nutritional deficiencies such as calcium and vitamin D. Multifaceted treatment of AN is required to restore bone mineral density to the normal range.
The electrolyte disturbances of AN and BN depend on whether purging is primarily by vomiting or by abuse of laxatives or diuretics (or both) and can be life threatening. The medical management of these cases can be complex and must be individualized. Refeeding syndrome may occur in severely malnourished patients as a result of severe shifts in fluids and electrolytes, in particular phosphate, from extracellular to intracellular spaces. These electrolyte shifts can lead to cardiovascular, neurologic, and hematologic complications and can be associated with significant morbidity and mortality.83 Comerci72 presented detailed case histories of an anorexic and a bulimic patient, including critiques of their treatment, which highlight the intricacies of successful medical management of these disorders.
Neuroimaging
This is a growing area of eating-disorders research supported by major improvements in imaging technology.84,85 In AN, enlargement of cortical sulci and subarachnoid spaces suggestive of cerebral, and on occasion cerebellar, atrophy have been reported, the changes being positively correlated with poor neuropsychological test performance and being reversible to varying degrees with weight gain.86–88 Reduced gray matter volume in the anterior cingulate cortex has been reported in AN compared to healthy controls, the amount of the decrease being correlated with illness severity.89 Studies in recovered AN and BN patients have differed, showing either persistent alterations or normalization of gray- and white-matter volumes.90,91
With regard to CNS metabolites, proton magnetic resonance spectroscopy (1H-MRS) indicates higher ratios of choline-containing compounds to creatine and N-acetylaspartate, as well as reduced myoinositol, in the frontal white matter and occipital gray matter in association with decreased body mass index (BMI), suggesting starvation-associated increased cell membrane turnover.92 Consistent with these findings, 31P-MRS has shown altered phosphodiester and phosphomonoester peaks in malnourished anorexics, suggesting that reduced body mass alters CNS cellular membrane phospholipid metabolism.93 Reduced blood flow in frontal, parietal, and frontotemporal cortex, as shown by single photon emission computed tomography (SPECT), has been reported in AN, which reverted to normal perfusion with clinical remission.94 Another SPECT study suggested reduced blood flow in the anterior cingulate in patients with AN compared to controls, both before and after weight restoration.95
Functional neuroimaging (fMRI) also is delineating activation of limbic and paralimbic areas that may be involved with calorie fear, including exaggerated activation of the caudate and reduced activation of the insula to taste stimuli in women recovered from AN compared to normal women.96,97 These areas also have been implicated as neural substrates of obsessive-compulsive and depressive symptoms.98 Decreased food-stimulated activation of several cortical areas has also been shown by fMRI in chronically ill anorexics, compared to those exhibiting long-term recovery.99 Positron emission tomography (PET) imaging has demonstrated reduced 5-HT1A and 5-HT2A receptor binding in frontal, parietal, and occipital cortex in both AN and BN patients, which may persist after recovery in both conditions.8,100,101 This may tie in with the candidate gene studies of these receptors discussed earlier; correlative studies have yet to be undertaken.
Neuroendocrinology
Abnormal hormone profiles and responses to challenge are closely related to the “starvation” status of AN and BN patients.102–106 Hormone abnormalities also may be present (but to a lesser extent) in normal-weight women with BN. The presence of starvation in AN is evident from the weight loss, but it may not be recognized in normal-weight BN: Although bulimic women often maintain a normal weight, they do so by restricting food intake when not bingeing and purging, and they often have poorly balanced meals. Starvation-induced depletion of hepatic glycogen stores results in free fatty acids and ketone bodies replacing glucose as the primary energy source. This shift from glycogenolysis to lipolysis and ketogenesis is associated with an increase in free fatty acids and their metabolites. β-Hydroxybutyric acid levels are elevated in both AN and BN,107 indicating that bulimic patients are nutritionally depleted despite their normal body weight.108
The relationship of starvation and eating disorders to neuroendocrine function is most clearly seen for the pituitary-gonadal axis. As mentioned, secondary amenorrhea is one of the criteria for AN in postmenarcheal women,74 and oligomenorrhea occurs in about 50% of bulimics. Table 30-5 lists the major endocrine disturbances that occur in AN and BN.103,109–112 The secondary amenorrhea is a direct result of altered gonadotropin secretion. Serum sex hormone–binding globulin may be increased, and both estrogen and testosterone are decreased.113 As indicated in Table 30-3, there are physical signs of severe estrogen deficiency. The luteinizing hormone (LH) response to LH-releasing hormone stimulation is blunted, but the follicle-stimulating hormone response is usually normal.
Table 30-5. Neuroendocrine Disturbances in Anorexia Nervosa and Bulimia Nervosa
| Anorexia Nervosa | Bulimia Nervosa | |
|---|---|---|
| Pituitary-Gonadal Axis | ||
| Plasma gonadotropins (LH, FSH) | ↓ | ±↓ |
| Plasma estradiol | ↓↓ | ±↓ |
| Plasma testosterone | ↓ | ? |
| LHRH stimulation of LH | ↓ | ±↓ |
| LHRH stimulation of FSH | ↔ | ↔ |
| Pituitary–Adrenal Cortical Axis | ||
| Plasma and CSF cortisol | ↑ | ±↑ |
| Plasma ACTH | ↔ | ↔ |
| CSF ACTH | ↓ | ↔ (↓ when abstinent) |
| CSF CRH | ↑ | ? |
| CRH stimulation of ACTH | ↓ | ? |
| ACTH stimulation of cortisol | ↑ | ? |
| Dexamethasone suppression test | 50%-90% nonsuppression | 20%-60% nonsuppression |
| Pituitary-Thyroid Axis | ||
| Plasma Total T4 | ±↓ | ↔ (±↓ when abstinent) |
| Plasma T3 | ↓↓ | ↓ |
| Plasma Reverse T3 | ↑ | ↔ (↓ when abstinent) |
| Plasma TSH | ±↓ | ±↓ (↑ when abstinent) |
| CSF TRH | ↓ | ? |
| TRH stimulation of TSH | ↓ | ↓ |
| Other Neuroendocrine Axes | ||
| Growth Hormone | ↑ | ↑ |
| Prolactin | ↔ | ±↓ |
| Prolactin response to serotonergic challenge | ↓ | ↓ |
| Melatonin | ±↑ | ? |
ACTH, Adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; CSF, cerebrospinal fluid; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LHRH, luteinizing hormone–releasing hormone; T4, thyroxine; T3, triiodothyronine; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone; ↑, increased; ↓, decreased; ↔, unchanged; ?, insufficient or conflicting data.
Fig. 30-2 illustrates circadian serum LH profiles of girls at different pubertal stages and in a 21-year-old woman with AN of 14 months’ duration whose body weight was 60% of ideal weight.109 This patient’s menarche was at age 14, and during the period of weight loss, her LH profile was that of a prepubertal girl. BN patients also may have decreased gonadotropin secretion if they have lost 15% or more of their body weight, but they usually have normal circulating gonadotropins and continue their menses.
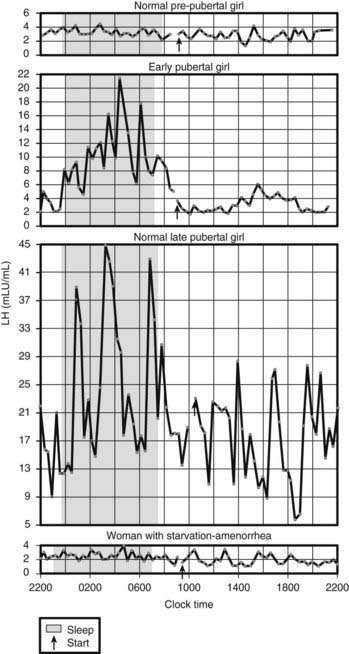
FIGURE 30-2. Circadian serum luteinizing hormone (LH) profiles in girls at different pubertal stages and in a 21-year-old woman with anorexia nervosa of 14 months’ duration whose body weight was 60% of ideal weight. Menarche was at age 14 years, and her LH profile is that of a prepubertal girl.
(From Vande Wiele RL: Anorexia nervosa and the hypothalamus. Hosp Pract 12:45–51, 1977.
With reference to the hypothalamic-pituitary–adrenal cortical (HPA) axis (see Table 30-5), the abnormalities in AN and in reduced-weight BN111,114,115 are strikingly similar to those occurring in 30% to 50% of patients with major depression.111,116,117 Circulating cortisol is increased at all times of the day and night, but the amplitude and timing of its circadian rhythm are preserved. Circulating adrenocorticotropic hormone (ACTH) is usually normal when determined by radioimmunoassay, as it is in major depression; the more specific immunoradiometric assay for ACTH1-39 has shown decreased ACTH in major depression,118 which also could be the case for ACTH1-39 in these eating disorders. Cerebrospinal fluid (CSF) ACTH concentrations appear to be decreased, but CSF corticotropin-releasing hormone (CRH) concentrations may be increased,111,115 as may be CSF vasopressin,111,119 a secretagogue for ACTH in addition to CRH,120 which appears to play a greater role in stress states than under normal conditions.121
Stimulation and suppression tests of the HPA axis have been conducted mainly in AN, and they are in accord with the baseline hormone findings. The ACTH response to CRH administration is reduced, undoubtedly secondary to enhanced negative feedback on the pituitary corticotrophs exerted by elevated circulating cortisol. The cortisol response to ACTH administration is increased, suggesting increased secretory capacity of the adrenal cortex. The low-dose dexamethasone (DEX) suppression test is abnormal in 50% to 90% of anorexics and in 20% to 60% of bulimics, depending on weight loss. Because DEX acts primarily at the pituitary, ACTH and cortisol escape from DEX suppression suggest increased suprapituitary stimulation of corticotrophs by CRH and vasopressin. CRH may be more influential than AVP in stimulating the HPA axis in AN, because the cortisol response to exogenous AVP administration is blunted.122 Taken together, the pituitary-adrenocortical findings indicate a mild to moderate activation of this hormone axis in AN and BN. As well, plasma neuroactive steroids are reported to be elevated in untreated women with both AN and BN.123
With reference to the pituitary-thyroid axis, starvation leads to considerably decreased plasma free triiodothyronine (T3) concentrations, along with somewhat decreased plasma free thyroxine (T4) and increased plasma reverse T3 concentrations. This represents the “euthyroid sick syndrome” hormone profile.124–129 The decreased circulating T3 helps reduce energy expenditure and minimizes muscle protein catabolism into amino acids for gluconeogenesis. CSF thyrotropin-releasing hormone (TSH) also appears to be reduced in AN.130 When bingeing, bulimic patients generally have normal thyroid indices with perhaps reduced T3 and TSH concentrations; however, when they stop bingeing, their pituitary-thyroid axis function resembles that of anorexic patients.131–133
Insulin-like growth factor type 1 (IGF-1) concentrations are low in both AN and BN, and circulating growth hormone (GH) is increased, likely owing to both diminished feedback of IGF-1 on GH secretion and primary hypothalamic dysfunction.134,135 Circulating prolactin is usually unchanged in AN and may be reduced in BN. Prolactin responses to serotonergic challenges are diminished in both AN and BN,136–142 suggesting decreased CNS serotonergic neurotransmitter function.101 (Serotonin uptake–inhibiting antidepressants have shown promise in the treatment of these eating disorders, as discussed later.) Circulating melatonin has been reported as both unchanged and increased in AN and as unchanged in BN.143–146
The effect of reduced caloric intake on these endocrine systems has been studied in healthy individuals.147,148 When healthy women were starved, plasma gonadotropin concentrations declined. The women also experienced increased circulating concentrations of cortisol and GH and decreased plasma T3 concentrations despite normal plasma T4—the “euthyroid sick syndrome” hormone profile. The endocrine abnormalities associated with starvation in the healthy female subjects reversed with the resumption of normal eating.
The endocrine changes in both AN and BN revert to normal with successful treatment of these illnesses, indicating that the endocrine changes are “state” markers of the metabolic stress of starvation and malnutrition. It should be emphasized that in addition to its effects on hormone secretion, starvation can lead to abnormal psychological states. Semistarvation of male conscientious objectors to military service was associated with increased irritability, labile mood, depression, decreased concentration, decreased libido, and decreased motor activity,149 and starvation and malnutrition can exaggerate the comorbid psychiatric symptoms of AN and BN. These changes reinforce the concept of starvation-related “state” changes influencing both the behavioral and the endocrine aspects of eating disorders.
Central Nervous System–Related Neuropeptides
Since the 1980s, the realization has evolved that the peripheral hormonal disturbances in AN and BN are a consequence of the malnutrition associated with starvation and bingeing, rather than being etiologic. Contemporaneously, an understanding of how CNS neuronal pathways contribute to starvation-induced alterations in peripheral hormonal secretion has developed. The mechanisms for controlling food intake and energy homeostasis involve a complex interplay among peripheral (taste, local autonomic influences on GI/neuropeptides, vagal afferent nerves) and CNS neurotransmitters and neuromodulators, including monoamines and neuropeptides that influence hunger and satiety.8,9,115,150 Compounds such as norepinephrine, serotonin, insulin, opioids, neuropeptide Y and YY, leptin, CRH, vasopressin, and the orexins/hypocretins contribute to regulating the rate, duration, and size of meals, as well as the selection of carbohydrates and protein.106,151–153
Neuropeptides were initially determined to be regulators primarily of hypothalamic functions such as food and water consumption and metabolism, sexuality, sleep, body temperature, pain sensation, and autonomic function. These compounds also have been localized to other areas of the CNS besides the hypothalamus and pituitary, where they appear to regulate complex human mental functions such as mood, obsessionality, attachment formation, and risk-taking and addictive behaviors. Some of the behavioral disturbances occurring during starvation therefore may be related to alterations in function of neuropeptides throughout the CNS. These peptide systems work together multiply in overlapping CNS pathways that influence the spectrum of energy balance states. Table 30-6 lists the major neuropeptide disturbances that occur in AN and BN.8,9,104,115,119,154–157 These changes represent an adaptive profile to weight loss specific to AN and are not present in constitutionally lean women.158
Table 30-6. Central Nervous System Neuropeptide Disturbances in Anorexia Nervosa and Bulimia Nervosa
| Anorexia Nervosa | Bulimia Nervosa | |
|---|---|---|
| CSF neuropeptide Y | ↑ | ↔ |
| CSF peptide YY | ↔ | ↔ ↑ During abstinence |
| Plasma ghrelin | ↑ | ↑ |
| Plasma leptin | ↓ | ↓ |
| CSF leptin | ↓ | ? |
| Plasma adiponectin | ↑ | ↑ |
| Plasma resistin | ↓ | ? |
| Plasma cholecystokinin | ? | ↓ |
| CSF cholecystokinin | ? | ↓ |
| CSF β-endorphin | ↓ | ↓ |
| CSF dynorphin | ↔ | ↔ |
| CSF vasopressin | ↑ | ↑ |
| CSF oxytocin | ↓ | ↔ |
↑, Increased; ↓, decreased; ↔, unchanged; ?, insufficient or conflicting data.
NEUROPEPTIDE Y AND PEPTIDE YY
These phylogenetically and structurally related peptides share the same receptor family (Y1, Y2, Y4, Y5) and are among the most potent stimulants of feeding behavior in animals, particularly for carbohydrate-rich foods.115 Neuropeptide Y (NPY) occurs in high concentrations in limbic structures, including the hypothalamus, and is present throughout the cerebral cortex.159 NPY-producing cells in the arcuate nucleus of the hypothalamus co-express agouti-related protein and are inhibited by leptin. NPY increases during hunger, falls during meals, and acts on the paraventricular nucleus to mediate increased eating and reduce energy expenditure. Its effect is counteracted by CRH, and a dynamic equilibrium between NPY and CRH neuronal activity appears to be an important regulator of food intake. The Y1, Y2, and Y5 subtypes of NPY receptors have been implicated in the regulation of feeding.159
In contrast, peptide YY (PYY) occurs in lower concentrations in the CNS, caudal brainstem, and spinal cord. PYY occurs in two forms and is primarily located in endocrine cells of the lower GI tract, where it helps mediate motility and function. PYY1-36 is strongly orexigenic. PYY3-36, on the other hand, has particular affinity for the Y2 receptor, it increases in response to meals, and its actions are to decrease appetite and reduce food intake.160
Intracerebroventricular (ICV) NPY administration in animals produces many of the physiologic and behavioral changes associated with AN, including gonadal steroid-dependent effects on LH secretion, suppression of sexual activity, increased CRH in the hypothalamus, and hypotension.115 Underweight AN patients have elevated CSF NPY, likely a result of their malnourished status. In contrast, such patients, whether underweight or recovered, have normal CSF PYY concentrations. CSF NPY returns to normal with recovery, although patients with amenorrhea may continue to have higher CSF NPY concentrations. Elevated CSF NPY is not an effective stimulant of feeding in underweight anorexics, as evidenced by their resistance to eating and weight gain. Anorexics typically display an obsessive and paradoxical interest in dietary intake and food preparation, and it may be that increased NPY activity in extrahypothalamic areas of the CNS contributes to these cognitions and behaviors.
ICV PYY administration in rats causes massive food ingestion to which tolerance does not develop, which prompted speculation that increased CNS PYY activity may contribute to bulimia. CSF PYY concentrations in normal-weight bulimic women when bingeing and vomiting were similar to those of controls,115 whereas CSF PYY was significantly elevated in bulimic women after a month of abstinence from bingeing and vomiting, compared to healthy volunteer women and AN patients. CSF NPY concentrations were normal in the bulimic women, in contrast to the elevated CSF NPY concentrations in the anorexic women. Plasma NPY and PYY concentrations in AN and BN patients, however, do not necessarily accord with CSF concentrations of these peptides and may have peripheral effects unrelated to the CNS.9 For example, AN patients may have normal CSF PYY concentrations, as noted earlier, but they also may have elevated plasma PYY concentrations, which can contribute to the osteoporosis noted in these individuals.79–82
It is not known why CSF PYY is normal in bulimics with chronic bingeing and vomiting and becomes elevated after a period of abstinence. Whatever the cause, this disturbance is of potential importance. Normal-weight bulimia carries a high recidivism rate despite treatment. Abnormally elevated CNS PYY activity in the abstinent bulimic may contribute to a persistent drive toward bingeing, particularly a desire for sweet, high-caloric foods.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree