9
Although minimally invasive thermoablative therapies have been most extensively described as a means to treat solid organs within the chest and abdomen, clinical studies and experience have suggested a role for percutaneous ablation in a multitude of organs, soft tissue, and skeletal sites throughout the body. As esoteric indications for the use of radiofrequency (RF), microwave, and cryoablation expand to include the adrenal gland, recurrent and advanced malignancies of the head and neck, soft tissue and bony lesions of the pelvis, as well as breast cancers, patients may seek palliation, a minimally invasive alternative to surgical intervention, or treatment for disease previously considered refractory to treatment. With further investigation and creative treatment strategies, thermal ablation will continue to be an integral component of oncology treatment plans as the fourth arm of cancer therapy.
♦ The Adrenal Gland
Patient and Tumor Selection
Primary neoplasms of the adrenal gland include the following: adrenal cortical carcinoma, adenomas (nonfunctioning or cortisol-producing), aldosteronomas, and pheochromocytomas. Traditionally, treatment options have included either open or laparoscopic surgical resection.1–7 The adrenal gland is also the fourth most common site for metastatic disease following the lung, liver, and bone, with contributory primary malignancies including lung, breast, skin (melanoma), kidney, thyroid, and colon cancers.8 The incidence of metastasis is specific to the primary tumor, with approximately 30 to 40% of lung and breast cancers, 50% of melanomas, and 10 to 20% of renal and colorectal malignancies likely to cause adrenal metastases.8 Similarly, surgical resection has been proposed as the treatment of choice for isolated adrenal metastatic disease,9,10 with a median survival of 28 months reported by Sarela et al11 status post–adrenalectomy for isolated adrenal metastasis. Paradoxically, patients with advanced stages of inoperable non–small cell lung cancer metastatic to the adrenal gland have also undergone adrenalectomy with hopes of increasing long-term survival rates.12
Less invasive treatment options such as selective arterial embolization and injection with alcohol or acetic acid have been described.13–16 As the arterial supply of the adrenal gland is threefold in anatomic design, catheterization of these vessels and thorough embolization of the gland remain challenging, and ethanol and acetic acid injections have shown to be insufficient when treating sizable lesions.17 Cryoablation was subsequently presented as a surgical alternative with use documented in both animal studies and in a patient with bilateral adrenal hyperplasia.18 With much literature and clinical practice documenting radiofrequency ablation (RFA) as a safe and effective minimally invasive ablation modality in the liver, lung, kidney, bone, and other organs, a role for RFA in the treatment of adrenal neoplasms was suggested.19–23
Adrenal Cortical Carcinoma
Adrenal cortical carcinoma (ACC), a primary malignancy arising from the adrenal cortex, is relatively rare with an annual incidence of 1 to 2 per 1,000,000 in the United States.24 Radiation and chemotherapy have not proven to be effective treatment options, and thus the mainstay of treatment is surgical resection despite the fact that there is a high rate of local recurrence often requiring repeat intervention.24 Wood et al,25 however, report on the feasibility, safety, and efficacy of using RFA in the treatment of recurrent primary ACC and its metastases in eight patients. A total of 15 lesions were ablated ranging in size from 1.5 to 9.0 cm, demonstrating that RFA was associated with minimal morbidity and was effective in the local control of both recurrent ACC, especially lesions <5 cm in diameter, and its metastases.
Adrenal Metastases
At our institution, percutaneous computed tomography (CT)-guided RFA was initially used 6 years ago to treat 13 solid adrenal masses in 12 patients ranging in age from 40 to 77 years (mean age 58 years).26 All patients were deemed nonsurgical candidates on the basis of medical comorbidities, concurrent disease at distant sites, or refusal of resection. Eleven lesions were metastatic from a variety of primary malignancies (five from lung cancer, four from renal cell carcinoma, and two from melanoma). At the time of RFA, metastatic disease was localized, affecting only the adrenal gland in six of the 11 cases (one patient with bilateral isolated adrenal metastases), and five cases were associated with the presence of additional distal organ metastases successfully controlled by chemotherapy, radiation, or prior resection. Tumor diameters ranged from 1 to 8 cm (mean 3.9 cm).26 To date, clinical experience at our institution continues to show that patients with metastatic disease to the adrenal gland are candidates for thermal ablation, which offers satisfactory local control rates in this population of patients.
Aldosteronomas and Pheochromocytomas
Of the two remaining lesions treated in the Mayo-Smith et al cohort, one patient had been diagnosed with an aldosteronoma and the other with a pheochromocytoma, for each of which laparoscopic surgical resection is the standard therapy, with reported associated morbidity rates of 7.5 to 23%.26–28 It is estimated that in 80% of patients with primary hyperaldosteronism the causative etiology is an aldosterone-secreting adrenal cortical adenoma with autonomous function.24 At presentation, the resulting clinical syndrome of hyperaldosteronism includes symptoms of diastolic hypertension, metabolic alkalosis, and hypokalemia, serving as the cause of ≤1% of clinically diagnosed hypertension.29
In the majority of patients, aldosteronomas are unilateral, affecting a single adrenal gland, and small, with approximately 20% found to be <1 cm, making them challenging to identify on cross-sectional imaging.24 The work of Mayo-Smith and Dupuy26 provides a clinical correlation for these observations. The study patient diagnosed with an adrenal aldosteronoma had clinical hypertension, a 1-cm mass on the left adrenal gland, and elevated serum aldosterone levels that lateralized to the left adrenal at selective venous sampling. This patient required oral potassium supplementation prior to ablation, consistent with a clinical finding of hypokalemia.
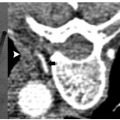
Pheochromocytomas (Fig. 9.1) are rare, hormonally active, neuroendocrine tumors that originate from the chromaffin cells of the adrenal medulla. The excessive secretion of catecholamines, namely adrenaline and noradrenaline, gives rise to clinical symptoms, including headaches, paroxysmal hypertension, diaphoresis, tachycardia, and anxiety. Diagnostic studies include measurement of serum metanephrines and, if necessary, a 24-hour urine collection for catecholamines, vanillylmandelic acid, and metanephrines. Only after a biochemical abnormality is proven with laboratory data should diagnostic imaging be performed.
As a clinical correlate, one of the 12 patients in the Mayo-Smith and Dupuy26 cohort had been diagnosed with a pheochromocytoma after biochemical evaluation, and diagnosis was histochemically confirmed on biopsy prior to ablation. An additional case series by Cheah et al30 documents RFA of a 68-year-old man with a 2-cm right adrenal pheochromocytoma deemed to be a high-risk surgical candidate and a 65-year-old man with a 3.3-cm right adrenal pheochromocytoma with an extensive cardiac history.30 It is standard of care that these patients receive preoperative alpha- and beta-blockade prior to surgical resection, routinely prescribed at our institution in the form of oral phenoxybenzamine and atenolol for 3 weeks prior to ablation, providing alpha- and beta-blockade, respectively.
Anatomic Considerations
The adrenal glands are suprarenal in position lying between T11 and L2, with the right adrenal posterior to the inferior vena cava and the left in close proximity to the aorta and adjacent pancreas. Fatty tissue encases each adrenal, forming an adipose capsule and branching fibrous extensions, which penetrate into the gland, resulting in a relatively fixed anatomic location. The rib cage and lungs extend caudally anterior to retroperitoneal adrenals with several centimeters of overlap. It is noted that during inspiration the distance between the adrenals and kidneys becomes more pronounced.
Patients are routinely positioned on the CT gantry bed, depending on the sidedness of the lesion to be treated. The ipsilateral decubitus position was found to be most advantageous when ablating relatively small adrenal lesions. When combined with a posterior percutaneous approach, this position made the adrenal readily accessible while compressing the ipsilateral lung by placing it in a dependent position. Thus, a trajectory could be chosen requiring minimal if any traversement of pulmonary parenchyma. When treating larger adrenal lesions, however, patients are routinely placed in the prone position.
When performing thermal ablation of the adrenal gland, care must be taken to protect the surrounding organs from thermal injury. The kidneys, stomach, pancreas, and liver lie in close proximity to the adrenals and are susceptible to injury, necessitating the use of thermoprotective strategies to isolate the adrenal gland during thermal ablation. For example, hydrodissection requires a 5% dextrose solution to be instilled adjacent to the adrenal gland by means of a CT-fluoroscopically placed catheter until vulnerable, surrounding structures are adequately displaced.31 Additional reported techniques utilize carbon dioxide in a similar manner.32
Technique
As the adrenal gland actively produces and stores catecholamines as well as cortisol, androgens, and mineralocorticoids, ablation of this gland poses the risk of releasing large amounts of both stress and steroid hormones into the bloodstream in a short period of time. Sudden exposure to high levels of circulating catecholamines places the patient at risk of developing rapid-onset tachycardia and hypertension. Thus, patients may be prophylactically treated with alpha-and beta-blockade prior to adrenal ablation.
Despite the fact that pharmacologic blockade is the standard of care prior to ablation of a pheochromocytoma, a controversial debate arises regarding pretreatment of patients who are to undergo ablation of adrenal metastases. Most interventional radiologists do choose to pretreat, as there is no scientific way to predict preprocedurally which patients are most at risk for an intraablative hypertensive crisis.24 At our institution, patients scheduled to undergo ablation of adrenal metastases are premedicated with phenoxybenzamine for 2 weeks prior to the procedure in an effort to provide alpha-adrenergic blockade. Pharmacologic beta-blockade may be simultaneously required in some patients due to resultant alpha-blockade–induced tachycardia, and thus is routinely prescribed in the form of atenolol.
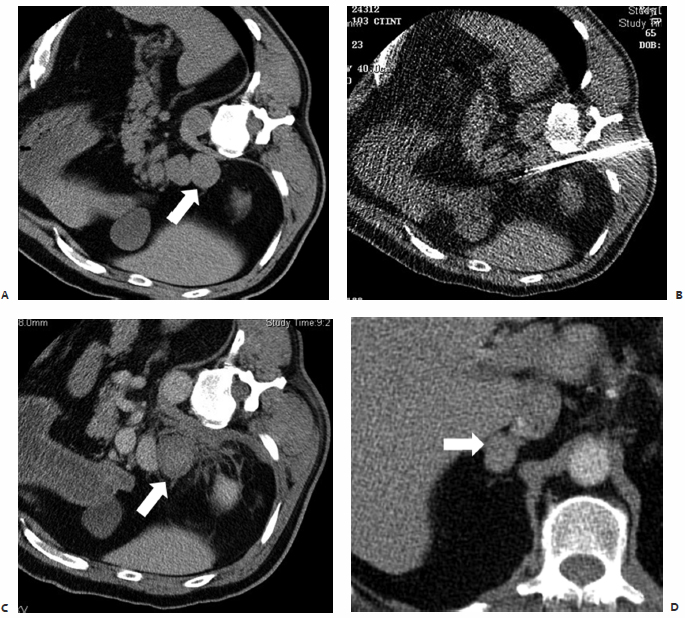
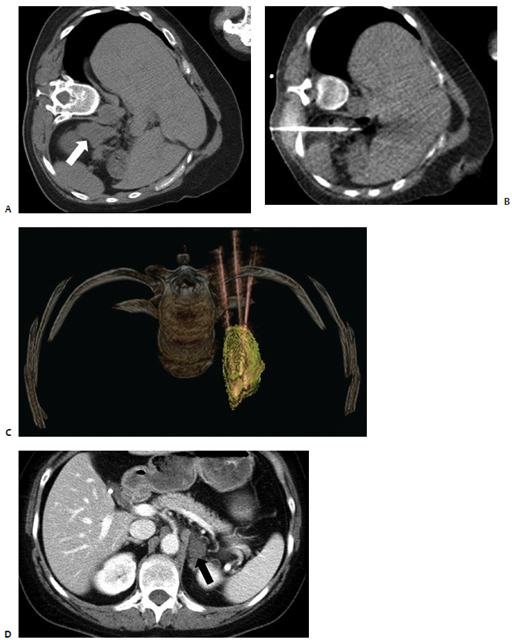
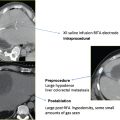
Fig. 9.1 A 65-year-old man was found to have a 3.3-cm mass at the apex of the right adrenal gland, episodic fluctuations in blood pressure, occasional syncope, tremors, headaches, and abdominal pain as well as a positive 24-hour urine epinephrine and metanephrine assay confirming the diagnosis of a right-sided pheochromocytoma. Due to the patient’s medical comorbidities and poor surgical candidacy, percutaneous radio-frequency ablation (RFA) was suggested at the discretion of the treating surgeon and radiologist. During the 8 weeks prior to ablation, the patient was treated with pharmacologic alpha-blockade. Intraprocedurally, the patient was placed in the right lateral decubitus position, and general anesthesia was administered by an anesthesiologist. (A) Computed tomography (CT) scan prior to treatment showing the mass at the apex of the right adrenal gland (arrow). (B) Under CT-fluoroscopic guidance, a Cool-Tip cluster electrode (Valley Laboratory, Boulder, CO) was placed intralesionally and a total of five applications were performed, each 7 to 12 minutes in duration. Fluctuations in blood pressure were treated at the discretion of the anesthesiologist and radiologist with intravenous nitroprusside. (C) Immediate, postablation contrast-enhanced CT demonstrates central hypodensity (arrow) consistent with coagulation necrosis and no enhancement indicative of residual disease. The patient was admitted for overnight observation and discharged the following day without complication. The patient’s blood pressure and urine metanephrines normalized over the course of several weeks allowing the cessation of pharmacologic therapy. (D) Contrast-enhanced CT scan 1 year after RFA shows a 75% reduction in tumor volume and lack of enhancement (arrow), consistent with successful treatment.
The standard departmental RFA protocol is followed in the treatment of patients undergoing adrenal ablation. All patients routinely undergo staging CT of the chest, abdomen, and pelvis prior to ablation as part of the pretreatment outpatient workup. Neither prophylactic nor intraprocedural antibiotics are routinely administered. Percutaneous ablation treatments are performed under conscious sedation administered by a dedicated nursing professional who continuously monitors electrocardiogram (ECG) tracings and vital signs. If deemed necessary, general anesthesia is used with continuous blood pressure monitoring via an arterial line. In cases at our institution in which a pheochromocytoma is to be ablated, phar-macologic sedatives are administered by an anesthesiologist with prior experience in caring for patients undergoing surgical resection of such lesions.26 Intraprocedural hypertension is a concern, and is reported by Cheah et al30 in a patient who required intravenous nitroprusside during ablation.
Based on lesion size, three-dimensional configuration, location, and proximity to or involvement of neurovascular structures, the most suitable ablative modality, RF, microwave, or cryoablation, is used. When using an RF system, a deployable array, cluster electrode, or switching controller is selected for use, enabling the creation of a larger ablation zone while reducing the number of applications required per session. (An application or treatment is the placement of electrodes and application of RF energy, and a session is a patient’s visit to the radiology suite for an ablation procedure.) For all adrenal lesions >3 cm in diameter, we prefer to use either microwave or cryoablation. With the flexibility of using multiple antennae/probes simultaneously in customized three-dimensional arrays, improved coverage of relatively larger lesions is made possible.
Postablation Management, Efficacy, and Follow-Up
The first several patients to undergo percutaneous adrenal RFA at our institution were admitted for overnight observation due to a lack of clinical data on procedural tolerability.26 As there were no major complications or episodes of postablation hypertension, the majority of ablations thereafter were performed on an outpatient basis. Patients were discharged postprocedurally in accordance with the standard departmental protocol. Postablation discomfort was treated with acetaminophen or acetaminophen/hydrocodone. Patients were routinely seen for outpatient follow-up within 2 weeks. Contrast-enhanced CT scans were to be performed at 3- and 6-month intervals from the date of ablation to rule out the presence of residual or new adrenal enhancement indicative of residual or recurrent disease, respectively.
In the study by Mayo-Smith and Dupuy,26 patients were followed for an average of 11.2 months (range, 1 to 46 months) during which time 11 of 13 treated lesions, 85%, revealed neither clinical nor radiographic evidence of residual or recurrent disease, thus indicating complete tumor necrosis and no need for re-treatment (Fig. 9.2).26 A remaining two of 13 lesions were found on follow-up imaging to demonstrate small areas of residual enhancement within the ablation zone, consistent with residual disease. The average diameter of these index lesions was 6.0 cm compared with an average of 3.6 cm among the aforementioned 11 masses. Although not found to be statistically significant, larger lesions were thought to be associated with a higher incidence of residual disease, as the available technology at the time of this study allowed for the treatment of a lesion only ≤5 cm in each single application.26
Similar efficacy of RFA in the treatment of adrenal metastases was shown in Italy. Carrafiello et al17 percutaneously ablated seven adrenal lesions (including the re-treatment of a single lesion with residual disease) ranging in size from 1.5 to 4.0 cm with an average of 2.9 cm. Only one patient developed severe intraprocedural hypertension requiring pharmacologic treatment with beta-blockade. Similar to the outcome at our institution, five of the six lesions revealed no evidence of residual disease on follow-up imaging, and effective local control was demonstrated, especially in those patients with lesions <5 cm in diameter.17
The potential biochemical sequelae of adrenal RFA were illustrated by the patient with bilateral solitary adrenal metastases who underwent two separate ablation sessions at our institution.26 Prior to initial ablation and after treatment of the left adrenal lesion, this patient underwent adrenal function tests. Cosyntropin stimulation tests and renin levels were normal on both occasions, signifying normal glucocorticoid and mineralocorticoid function. Following ablation of the right adrenal lesion, these tests were again performed and found at this time to be significantly abnormal, indicative of thorough ablation of all adrenal parenchyma. Prior studies have estimated that more than 90% of all adrenal tissue must be rendered nonfunctioning before biochemical function is compromised.33 Thus, this patient was pharmacologically managed with oral glucocorticoid (prednisone) and mineralocorticoid (fludrocortisone acetate) replacement therapy.
Laboratory profiles should be routinely monitored following RFA of biochemically functioning tumors. Serum hormone levels and abnormal biochemical activity are expected to normalize by 1 week, provided that the lesion was successfully treated.24 Serum potassium levels should be closely followed after aldosteronoma ablation. Mayo-Smith and Dupuy26 showed that after RFA of an adrenal aldosteronoma, serum aldosterone levels normalized, and the patient was able to discontinue the use of all oral potassium supplements as no clinical evidence of hypokalemia remained. Similarly, the patient with a biopsy-proven pheochromocytoma was able to discontinue the use of all pharmacologic antihypertensives following ablation and remained normotensive. In correlation with this clinical finding is the lack of significant enhancement of the adrenal mass seen on 1-month follow-up CT images. Importantly, all antihypertensive medications in those patients undergoing ablation of an aldosteronoma or pheochromocytoma should be continued in the immediate postablation period and then gradually tapered under medical supervision.
♦ The Thyroid Gland
Patient and Tumor Selection
The American Cancer Society estimated that there would be over 37,000 new cases of thyroid cancer in the United States in 2009. Although thyroid cancer has a low associated mortality and a collective 5-year survival of 97%, approximately two thirds of patients are between the ages of 20 and 55 at the time of diagnosis.34 To date, the most common subtype of well-differentiated thyroid cancer remains papillary carcinoma, accounting for 80% of all thyroid malignancies. The natural history of papillary carcinoma is that of slow-growing lesions commonly localized to a single lobe of the thyroid gland, with occasional bilobar involvement and frequent metastases to lymph nodes within the neck.34
Fig. 9.2 A 47-year-old woman with stage IV non–small cell lung cancer status posttreatment with cisplatin, etoposide, and radiation to the midthorax, presents for percutaneous microwave ablation (MWA) of a 4.4 × 2.3–cm metastatic lesion at the left adrenal gland. Intraprocedurally, the patient was placed on the CT gantry table in the left lateral decubitus position and general anesthesia was induced. (A) CT scan prior to treatment showing the metastatic lesion at the left adrenal gland (arrow). (B,C) Under CT-fluoroscopic guidance, with continuous blood pressure monitoring via arterial line, three 17-cm microwave antennae with 3.7-cm active tips were placed intralesionally in a three-dimensional triangular array spaced 2 cm apart. A single 4½-minute application was performed. Systolic and diastolic blood pressure values were observed to double, requiring IV pharmacologic intervention. However, there were no additional complications, and the patient was discharged from the radiology suite 4 hours after the procedure. (D) One-year follow-up CT reveals a stable postablation zone within the left adrenal gland (arrow), with no evidence of pathologic enhancement.
The standard treatment of papillary carcinoma is surgical resection of the thyroid gland. Either a total or subtotal thyroidectomy is performed, with resection of all suspicious lymph nodes within the central compartment of the neck. If metastasis to lymph nodes within the lateral compartment of the neck is suspected, modified radical neck dissection is also indicated at the time of initial surgery. With recurrence rates reaching 20.5% at a mean follow-up of 11.3 years, patients with papillary carcinoma are routinely followed postoperatively by ultrasound (US) examination of both the central and lateral compartments of the neck in conjunction with serum thyroglobulin testing.35 Factors predictive of recurrence include diagnosis at a young age, increased size of the primary neoplasm, extracapsular spread, and the presence of distant metastatic disease most commonly in the lungs or bone.36,37
Recurrent disease is most likely found within the surgical bed or lymph nodes of the central or lateral compartments.38 Traditionally, these patients with recurrent well-differentiated thyroid carcinoma identified on US are taken back to the operating room for reexploration and resection of affected tissue or lymph nodes. However, in patients who have undergone a prior neck dissection, normal tissue planes in the central and lateral compartments are distorted due to scar tissue formation, and reoperative surgery is associated with higher complication rates.39 Recent work at our institution demonstrates that percutaneous RFA may be an effective and relatively safe alternative to surgical management.39,40
In the first reported study documenting the use of RFA in patients with localized recurrent well-differentiated thyroid carcinoma, Dupuy et al40 demonstrated the percutaneous use of RFA in the treatment of eight patients with biopsy proven recurrence within the neck (Fig. 9.3). Six patients presented with recurrence in the lateral compartment and two with disease in the central compartment. Lesions averaged 2.4 cm in size, ranging from 0.8 to 4.0 cm, with a mean tumor volume of 3.7 mL. Subsequent work by Monchik et al39 reported on an expanded cohort of such patients, bringing the total to 16. As detected by US examination, three patients presented with a recurrent mass within the surgical bed, and the majority, 13 patients, presented with lymph nodes in the lateral neck found to be positive for recurrence on US-guided fine-needle aspiration (FNA) biopsy.39
Twelve of these 13 patients had undergone a modified radical neck dissection at the time of initial surgery. Dupuy et al40 recommend that RFA be used to treat patients with a local recurrence in the lateral compartment only after the patient has undergone a modified radical neck dissection. If a positive node is found within a previously unexplored lateral compartment, chances are there is more than a single affected node, and surgical intervention is appropriate prior to ablation. Exceptions may be made if only one or two sites are identified, both of which are amenable to percutaneous ablation. Pathology was consistent with papillary carcinoma in 15 patients, with one reported case of medullary thyroid carcinoma. A paratracheal lymph node and nodes in close association with the right common carotid artery were treated as well as nodes within the right submandibular region, jugular chain, retroauricular space and bilateral supraclavicular regions, thyroid beds, and lateral neck compartments.40
Anatomic Considerations
The thyroid gland is anatomically situated within the anterior aspect of the lower neck and consists of two lateral lobes extending from the fifth cervical to the first thoracic vertebra connected medially by the isthmus. Arterial blood is supplied by the superior and inferior thyroid arteries and venous drainage by the superior, middle, and inferior thyroid veins. In close association with the inferior thyroid artery is the recurrent laryngeal nerve, a branch of the vagus nerve that provides motor and sensory innervation to the larynx. The left and right recurrent laryngeal nerves descend into the thorax, with the left looping under the aortic arch and the right under the subclavian artery, before coursing cranially within the tracheoesophageal groove to innervate the laryngeal muscles of the neck. Positioned on both sides of the trachea, and immediately posterior to the thyroid gland, the recurrent laryngeal nerve is vulnerable to mechanical injury during surgical dissection as well as thermal injury during RFA.
The treating radiologist should exercise extreme caution when ablating lesions in the lateral aspect of the central compartment due to the close proximity of the recurrent laryngeal nerve. As a precautionary measure against thermal nerve injury, hydrodissection may be used in a manner similar to that described previously for adrenal ablation. Under image guidance, a solution of 5% dextrose in water may be injected between the target lesion and expected anatomic location of the nerve acting to physically displace the nerve and create a protective thermal barrier from the delivered RF energy.39 Thermally induced damage to blood vessels is not a concern due to the well-described vessel-mediated cooling or “heat-sink” effect associated with RFA.
Technique
All patients scheduled to undergo ablation at our institution are routinely seen in outpatient consultation, and the standard departmental RFA protocol is followed in the treatment of all patients who undergo ablation of local recurrent well-differentiated thyroid cancer. Unique to this patient population, as documented in the studies by Dupuy et al40 and Monchik et al,39 preablation workup includes US-guided FNA biopsy in all patients for pathologic confirmation of recurrent disease. Intraprocedurally, grounding pads are placed on all patients in accordance with the manufacturer’s specifications. Conscious sedation is administered by dedicated nursing professionals, who continuously monitor ECG tracings and vital signs.
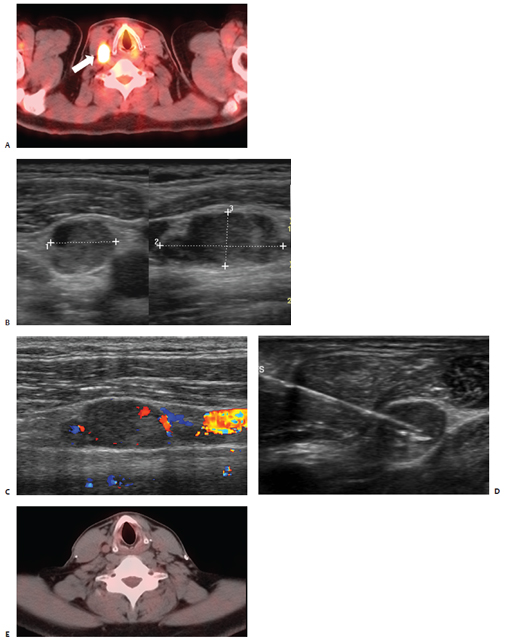
Fig. 9.3 A 43-year-old man status post–total thyroidectomy with selective neck and paratracheal node dissection as well as radioactive iodine therapy for papillary carcinoma with positive nodes in the lateral and central compartments presented 5 years later with biopsy proven cervical node recurrence, prompting a right modified neck dissection. (A) Follow-up PET-CT revealed intense uptake within a right jugulodigastric lymph node (white arrow). US examination (B) confirmed the presence of an enlarged lymph node in the lateral compartment of the right neck beneath the sternocleidomastoid muscle at the level of the true vocal cords measuring 1.7 × 0.9 × 0.75 cm with evidence of flow on Doppler interrogation (C). One week later, the patient presented for percutaneous ablation of recurrent nodal disease. (D) Under US guidance, a 15-cm RF electrode with a 1-cm active tip was positioned in the inferior aspect of the target lesion in close proximity to the carotid artery and jugular vein. Within 1 minute, a maximum temperature of 65°C was attained, and the electrode was then repositioned superiorly for a second application. (E) Two-year follow-up PET-CT demonstrates a normal distribution of activity within the ablation zone and no evidence of fluorodeoxyglucose-avid disease.
All procedures are performed under real-time US guidance (10 to 14 MHz linear US transducer) enabling proper intra-lesional placement of a single internally cooled RF electrode with an active-tip length of 1 or 2 cm (Cool-Tip; Valley Laboratory/Covidien, Boulder, CO) powered by an RF generator (Cosman Coagulator-1, Valley Laboratory/Covidien). Target lesions are treated with the maximum allowable current for 2 to 12 minutes, and cytotoxic temperatures are maintained at 50° to 90°C for a minimum of 2 minutes to ensure adequate induction of cytotoxic conditions. Multiple RF applications are performed per session at the discretion of the treating radiologist. The largest lesion in the Dupuy et al40 series measured 4 cm and required five individual applications. Postablation electrode temperature readings >50°C as well as real-time intraprocedural observation of micro-bubble formation and loss of intralesional vascular flow on color-Doppler images served as session end points indicative of appropriate thermocoagulation.
Postablation Management, Efficacy, and Follow-Up
All patients in studies by both Dupuy et al40 and Monchik et al39 who underwent percutaneous RFA of local recurrent well-differentiated thyroid carcinoma in either central or lateral neck compartments were monitored in the postprocedure recovery area until all sedative effects resolved. Patients were discharged in accordance with the department’s protocol within 2 to 6 hours. Postprocedural discomfort was treated with acetaminophen or hydrocodone/acetaminophen. Routine follow-up included a 1- to 2-week postprocedural visit and thereafter regularly scheduled outpatient visits to the tumor ablation clinic. Patients were monitored by means of physical examination, serum thyroglobulin laboratory values (calcitonin levels in the case of medullary carcinoma), and high-resolution ultrasonography at 6-month intervals.
In the initial work documented by Dupuy et al,40 all eight patients tolerated thermal ablation to completion; several of the patients underwent treatment at multiple sites. One patient was found on follow-up examination to have suspicious findings, prompting biopsy, and the patient underwent reablation at that site. A second patient, who had undergone RFA of two individual recurrences at the time of initial ablation, was found at 2-year follow-up to have a recurrence at one of the ablated sites, which necessitated retreatment. One of the eight patients died of advanced systemic metastatic disease 10 months following ablation, and the remaining seven demonstrated no uptake in the neck on follow-up total body scans.40
A reduction in thyroglobulin levels was observed in six of the eight patients, and US examination at 3 months postablation showed loss of hypervascularity on color Doppler, which was present in seven patients prior to treatment. Following thermal ablation, ablated lymph nodes were no longer hypoechoic but rather echogenic relative to muscle, with a mean reduction in size from 2.4 to 1.8 cm and continued shrinkage at the 6-month follow-up.40 It is advised that any lymph nodes that are found in postablation imaging studies to have increased in size may harbor residual tumor, and they require US-guided FNA biopsy.
Additional data subsequently presented by Monchik et al39 showed no evidence of recurrent disease at the site of RFA in 14 of the 16 treated patients at a mean follow-up period of 40.7 months. All 16 patients reported self-limited regional discomfort and neck swelling lasting only 1 to 2 weeks following ablation. No infectious complications were reported, nor burns at the sites of grounding pad placement. Procedure-related complications did, however, include a minor skin burn. The burn measured 0.5 cm in diameter and was located at the percutaneous RF electrode entry site due to contact of the proximal portion of the electrode active tip with the patient’s skin. A second patient who underwent RFA of recurrent papillary carcinoma in the central compartment, subsequent to total thyroidectomy, suffered permanent vocal cord paralysis due to thermal injury of the recurrent laryngeal nerve. This presented as audible hoarseness immediately following ablation, and despite symptomatic improvement over the following 2 months, laryngoscopic examination confirmed right vocal cord palsy.39
Further efficacy of RFA of local recurrent well-differentiated thyroid carcinoma is demonstrated by the work of Solbiati et al,41 who report on a series of two patients with papillary carcinoma metastatic to supraclavicular lymph nodes. Lesions ranging in size from 1.5 to 2.0 cm were ablated under US guidance using internally cooled electrodes, and complete necrosis without evidence of recurrent disease was found at ablation sites in both patients at 1-year follow-up.41 Thus, percutaneous RFA has emerged as a viable minimally invasive alternative to surgical management and high-risk reexploration in patients who have already undergone thyroidectomy (partial or complete), radioactive iodine ablation, and lymph node dissections. Ablated sites are amenable to future imaging and US-guided biopsy if indicated in longitudinal follow-up, and lesions <0.1 cm are identifiable. Unlike radioactive iodine, RFA may be repeated in an area of prior treatment as deemed necessary by the treating radiologist.
♦ Head and Neck Tumors
Patient and Tumor Selection
Worldwide incidence of head and neck cancers is reported to exceed 500,000 cases each year, with an annual incidence of 40,490 cases in the United States in 2006, accounting for approximately 3% of adult malignancies. An associated mortality of 11,170 was also reported in the U.S. that same year.42 Substantial morbidity is reported in these patients, who commonly suffer from intractable pain, cranial neuropathies, otalgia, odynophagia, dysphagia, trismus, and limited tongue mobility, making oral consumption of daily caloric requirements a challenge.43 It is estimated that two thirds of patients present with locally or regionally advanced disease, and with conventional treatment options, including surgery and adjuvant radiation therapy, 5-year survival rates average 55%.44,45 As speech and swallow mechanisms and functionality decline secondary to chemotherapy, intraarterial chemoinfusion, and radiation, patients commonly refuse additional necessary treatment. Compounding this are the poor rates of local control and survival as well as minimal improvement in quality of life. Additionally, only a small percentage of patients are surgical candidates due to extensive local disease, involvement of vital structures, and medical comorbidities. Thus, clinical studies propose that percutaneous RFA, a safe and effective minimally invasive treatment modality, may improve the quality of life in patients with head and neck cancers.
The work of Brook et al43 details the use of CT-guided RFA in the palliative treatment of 14 patients with recurrent advanced head and neck malignancies. All patients had previously undergone surgery, radiation, and chemotherapy, subsequently presenting with biopsy-proven stage IV disease. Palliation was sought for symptoms including pain, airway obstruction, dysphagia, and poor oral/jaw mobility—all secondary to aggressive tumor growth. Malignancies treated included squamous cell carcinoma of the tongue, tonsil, oropharynx, and maxillary sinus, as well as medullary thyroid carcinoma, basal cell carcinoma of the skull base, and an angiomatoid fibrous histiocytoma of the neck and posterior fossa. Lesion sizes ranged from 3.5 to 10.0 cm, with a mean of 6.3 cm.43
A case report by Bui and Dupuy46 documents the treatment of an adenoid cystic carcinoma of the salivary gland, a rare malignancy associated with poor patient prognosis and an aggressive natural history, with the potential for local extension and distant metastasis. As most lesions are in close proximity to vital neurovascular structures, wide local excision is usually not feasible, making reoperative intervention and radiation necessary due to frequent local recurrences. This symptomatic, recurrent adenoid cystic carcinoma in the right temporal bone extending posteriorly to abut the cerebellum measured 3 × 4 × 4 cm. There was associated destructive involvement of the petrous portion of the right temporal bone and right skull base.46
Anatomic Considerations
Both superficial and deep lesions of the head and neck are amenable to percutaneous ablation by means of various yet precise electrode trajectories.47 Factors to be taken into consideration include lesion size, location, three-dimensional shape, and proximity to or involvement of neurovascular structures and aerodigestive organs. Supra- or infrahyoid anatomic location of the target lesion is foremost in selecting a percutaneous electrode entry site and anticipated route. Suprahyoid lesions, including those located within the skull base and upper cervical vertebrae, may be accessed via a subzygomatic, retromandibular, paramaxillary, submastoid, transoral, or posterior approach. Infrahyoid lesions and those located within the lower cervical vertebrae are routinely accessed via the anterolateral, posterolateral, or direct posterior approach. More than one approach may be used during a single ablation session, with the RF electrode redirected for individual treatment applications.48
Technique
Brook et al43 reported that preablation imaging workup routinely included noncontrast and contrast-enhanced CT scans with additional contrast-enhanced magnetic resonance imaging (MRI) if deemed necessary. Intraprocedurally, general endotracheal anesthesia was used for all patients, and the oropharynx was packed with surgical gauze for electrical insulation if indicated. Intermittent CT scans were obtained both pre-and postapplication of RF energy, as CT-fluoroscopy was not utilized. Multi-tined RF electrodes were used, consisting of a 14-gauge, 25-cm needle cannula containing nine curved, flexible stainless steel electrodes that when deployed at the desired distance (up to 5 cm) took shape, forming an umbrella-like configuration.
Gas bubbles within the zone of developing necrosis were seen in the first five ablation sessions as a result of intratumoral temperatures exceeding 100°C, at which point water within tissues boils and vaporizes.49 Optimal conduction of delivered RF energy throughout the target lesion is negatively affected by the insulating effects of vaporized matter and gas. Thus, in all subsequent RF applications, target temperatures were reduced to 80° or 90°C from 105°C.43 Even heating of three-dimensional lesions was made possible by the use of this multi-tined electrode, enabling treatment of larger lesions with fewer adjacent/overlapping applications needed, thus shortening procedural times. Upon completion of ablation, the tines were retracted. As the RF probe was removed, the tract was cauterized by continuing to power the probe during slow withdrawal in an effort to limit postprocedural bleeding. Immediate postprocedure noncontrast CT scans were routinely obtained to rule out significant bleeding and damage to vascular structures or aerodigestive organs, and to serve as a baseline for which to compare future images. Contrast was administered in cases in which significant residual tumor was thought to be a concern.
In the case of the salivary gland carcinoma treated by Bui and Dupuy,46 with the patient under conscious sedation, two applications were performed using a single 3-cm, active-tip, internally cooled electrode repositioned before the second application. The two adjacent/overlapping treatment zones successfully encompassed the entire lesion. If in any case bony destruction was thought to be causing the patient’s discomfort, all tumor in the immediate vicinity of the skeletal structure was ablated. Relative to most soft tissues, cortical bone has poor electrical conductivity as shown by Dupuy et al,50 and thus it was challenging to reach and maintain temperatures above a cytotoxic threshold when an RF energy source was positioned adjacent to bone.
Postablation Management, Efficacy, and Follow-Up
Brook et al43 documented the treatment of 14 patients in 20 ablation sessions. Because of the morbidity within this population, patients were routinely admitted to the hospital postprocedurally, although one patient was discharged on the same day. After 47% of these ablation sessions, the patients were discharged after only one night; the rest were discharged after several days. Routine postablation follow-up included imaging studies, contrast-enhanced CT or MRI, as well as clinical assessment of palliative success.43
Improvement in quality of life was determined by using the University of Washington Head and Neck Quality of Life Questionnaire to evaluate pain, activity level, recreation, employment, disfigurement, speech, swallowing, chewing, and shoulder function.51 Four of the six patients who responded to the questionnaire (67%) reported an increase in quality of life following RFA. An additional eight patients (57%) reported subjective improvement in pain (n = 8), appearance (n = 1), and function (n = 4), including increased ability to swallow, breath, close one’s mouth, and relief due to immediate cessation of trismus.43 The patient treated by Bui and Dupuy46 reported resolution of facial pain and paralysis postprocedurally with no recurrence of symptoms during a remaining 18 months of life.
Three major periprocedural complications were reported within this cohort of patients.43 Complications included stroke, lethal pharyngeal hemorrhage secondary to pharyngocutaneous fistula and carotid blowout, as well as threatened carotid blowout treated with endovascular covered stent placement complicated by stroke. Retrospective analysis of intraprocedural CT images revealed that in each of these three cases either the RF cannula or retractable tines were within 1 cm of the carotid artery. Thus, Brook et al43 caution against the use of high RF energy levels in close proximity to great vessels, but conclude that RFA is a feasible and effective palliative treatment option.
Percutaneous RFA is an effective minimally invasive alternative to surgical resection for a variety of benign and malignant soft tissue masses in the head and neck, including hyperfunctioning parathyroid adenomas, lingual thyroid, laryngeal papillomata, and buccal lymphatic malformations.48,52–54
♦ Pelvic Malignancies
Patient and Tumor Selection
Pelvic malignancies collectively include gynecologic cancers of the ovary, endometrium, and cervix; genitourinary cancers of the bladder and prostate; as well as gastrointestinal cancers of the colon and rectum. Surgical resection, with or without adjuvant chemotherapy and/or radiation, remains the gold standard of treatment for these patients.55 However, local recurrence and metastatic foci remain a therapeutic challenge as additional surgical resection, radiation, and combined regimens are limited in both applicability and efficacy. Surgical resection of recurrent disease is potentially curative but only feasible in less than 50% of patients and carries associated morbidity rates as high as 32%.56 For those patients who are not surgical candidates, 5-year survival rates average 0 to 4%.57 If repeat resection and radiation fail to provide sufficient palliation of symptoms, very few therapeutic options remain.
With an aggressive natural history, rectal cancer is associated with a high rate of local recurrence, which, if left untreated, results in an average survival of 8 months.56 With involvement of neurovascular structures and medical comorbidities limiting resection of recurrent disease in salvage surgery, and chemoradiation able to provide only temporary palliation, with a 5-year survival of <5%, an optimal treatment modality for these patients remains yet to be found.58 In an effort to achieve long-term pain control, Belfiore et al58 treated 14 patients with severe and debilitating pelvic pain due to unresectable locoregional recurrence of rectal adenocarcinoma status post–abdominoperineal resection and radiation therapy with RFA. Similarly, Lefevre et al57 report on eight patients, six with unresectable recurrent rectal cancer, who underwent CT-guided RFA of soft tissue pelvic lesions for symptomatic relief and improved local control.
The remaining two patients presented for RFA of unresectable locoregional recurrence of a pelvic liposarcoma and uterine sarcoma initially treated by means of anterior resection with coloanal anastomosis and total hysterectomy, respectively. Both patients had also received adjuvant postoperative radiation.57 In addition to soft tissue lesions, painful bony metastases of both the pelvis and sacrum have also been treated with percutaneous RFA, as documented by Goetz et al.59 In this multicenter study, 24 patients with osteolytic bony metastases from colorectal, renal, and pulmonary primary malignancies underwent RFA after having received prior irradiation and being deemed poor candidates for further radiotherapy.59 Prior to the use of percutaneous thermal ablation, such patients were considered to have disease refractory to conventional treatment options.
Anatomic Considerations
Pelvic organs susceptible to infiltration with locoregional recurrent disease due to direct extension include the urinary bladder, rectum, and small bowel, as well as the uterus and vagina. In cases in which there is obvious involvement of these organs, the treating radiologist should proceed with caution. Prophylactic techniques such as intravesicular infusion of cold water during RFA of lesions involving the bladder should be routinely practiced.58 Locally recurrent disease also tends to be in close proximity to the pelvic sidewalls and internal iliac vessels due to total mesorectal excision indicated in surgical resection of malignancies involving the middle and lower one third of the rectum.57
It has been suggested that prior to percutaneous ablation an accurate measurement of the distance between the target lesion and any adjacent pelvic organs or neurovascular structures should be determined on pelvic MRI. If any single distance is <5 mm, RFA should be performed intraoperatively, either open or laparoscopically, to allow for proper dissection away from and protection of pelvic organs.57 Additionally, compared with traditional CT scanning, which has been shown to overestimate tumor volume due to poor differentiation among postradiation fibrosis, surgical scarring, and locoregional recurrence, positron emission tomography (PET)-CT has both a higher sensitivity and specificity. PET-CT enables accurate disease staging while preventing futile attempts at curative surgical resection.60
Technique
Patients in the Belfiore et al58 series routinely underwent contrast-enhanced CT or MRI scanning within 2 weeks prior to ablation to confirm the index lesion size and relation to adjacent pelvic structures. All ablations were performed under CT guidance, and patients received local anesthetics in addition to conscious sedation. Lesions <5 cm were ablated by means of a single 17-gauge cool-tip RF electrode, and lesions 5 to 7 cm in maximum diameter were ablated with a 15-gauge multi-tined expandable electrode, each placed via a transperineal approach. Regardless of the RFA system used, a single application was performed, and a target temperature of 90°C was reached and maintained for 9 to 12 minutes. Lefevre et al57 reported that one to three (on average, two) RF applications were performed, with an average duration of 12 minutes in the treatment of all eight lesions ranging in size from 2.0 to 4.5 cm (mean 3.3 cm) in diameter.58
At our institution, RFA has been used in both the treatment of pelvic soft tissue locoregional recurrences as well as palliation of bony lesions. If the chosen trajectory for percutaneous ablation requires traversement of intact cortical bone, an Ackermann needle is used to create a channel that may accept the RF electrode.55 When treating lesions of the pelvis and sacrum, Goetz et al59 document utilization of either conscious sedation or general anesthesia, as well as CT, US, or fluoroscopic guidance chosen at the discretion of the treating radiologist. A target temperature of 100°C was routinely reached and maintained for a minimum of 5 minutes, with a goal of 5 to 15 minutes. Lesions ≤3 cm were treated with single applications, and lesions >3 cm were treated with multiple applications and varied 3- to 5-cm deployable electrode arrays. If the lesion was >5 cm in diameter, the focal point for treatment was the margin between the lesion and involved bone, targeting the soft tissue/bone interface.59
With the advantage of offering pelvic and spinal joint preservation, cryoablation has also been used in the percutaneous treatment of both recurrent disease within the pelvis (Fig. 9.4) and giant cell tumors of the sacrum.61 A 7-cm soft tissue mass in the presacral coccygeal region, a biopsy confirmed recurrent rectal carcinoma, was treated with eight 2-mm cryoprobes placed percutaneously under CT-guidance. Two 10-minute freezes to −136°C with an intervening thaw were performed, resulting in visualization of a sufficiently sized ice ball, confirming appropriate coverage of the lesion.55
Postablation Management, Efficacy, and Follow-Up
Within 1 week from the date of ablation, patients in the Belfiore et al58 series were routinely seen in outpatient follow-up. At this time physical and neurologic examinations were performed, pain scale assessments made, and analgesic requirements assessed. Carcinoembryonic antigen laboratory values and trends were also monitored in subsequent visits. The recommended postablation imaging regimen consisted of contrast-enhanced CT or MRI obtained at 3, 6, 12, and 24 months.
Radiofrequency ablation is shown to be an effective palliative treatment option for patients with locoregional recurrence of rectal adenocarcinoma. Belfiore et al58 demonstrated a clinically significant reduction in pain in 13 of 14 ablated patients after 12 months, and longitudinally in 10 of 14 patients at 24 months. Interestingly, these results were obtained with radiographic evidence of complete ablation in only two patients, and ablation zones >75% of index tumor size in the remaining patients. Prior work by Ohhigashi and Watanabe62 had proposed that complete tumor ablation was essential when performing RFA for either palliative or curative intent in patients with recurrent rectal adenocarcinoma. During a follow-up period ranging from 6 to 36 months, four patients with solitary lesions, each less than 4 cm, treated with RFA and adjuvant chemotherapy, demonstrated complete ablation and resultant successful local control. For satisfactory RFA of lesions >4 cm in diameter, multiple applications per session with electrode repositioning were recommended.62
Of the eight patients treated by Lefevre et al,57 all patients were clinically pain free at 2-month follow-up. A highly significant reduction in pain scores and improvement in quality of life were also seen following RFA of bony lesions by Goetz et al.59 In this cohort of patients with disease deemed refractory to conventional treatment options, 95% of patients reported a clinically significant reduction in severe pain, occurring in 41% of cases within the first week following ablation. Opioid requirements at 8 and 12 weeks were also markedly reduced. It is theorized that heat generated during RFA may interrupt transmission of pain signals by destroying local sensory nerves while decreasing the production of cytokines used in nerve sensitization and osteoclastic stimulation by killing tumor cells.59
Radiofrequency ablation of recurrent pelvic malignancies, both soft tissue and bony lesions, is deemed to be a safe procedure. Reported complications most commonly include postprocedural bleeding, abscess formation at the treatment site (Fig. 9.5), transient bowel and bladder incontinence, and neuralgia.59,62–64 Lumbosacral radiculopathy with unilateral lower extremity involvement following ablation of the iliac bone and L4 vertebra in one patient and ablation of the right sacrum and ilium in a second patient have been confirmed on postablative electrodiagnostic studies.65 Despite evidence of axonal damage on electromyography, both patients demonstrated significant clinical improvement over the course of 1 to 7 months.65 inflammatory ureteric compression requiring endoscopic ureteral stent placement and formation of a colovesical fistula necessitating colostomy with transileal urinary diversion have also been documented.57
In addition to offering superior functional outcomes, due to preservation of pelvic and spinal joints, cryoablation results in low rates of both procedure-related adverse events and local recurrence when compared with other joint-preserving procedures. Malawer et al61 report on cryoablation used in the treatment of 102 patients with giant cell tumors of the sacrum. In follow-up, a minimum period of 4 years, both high cure rates and low complication rates were reported, with a <6% incidence of procedure-related pathologic fractures.
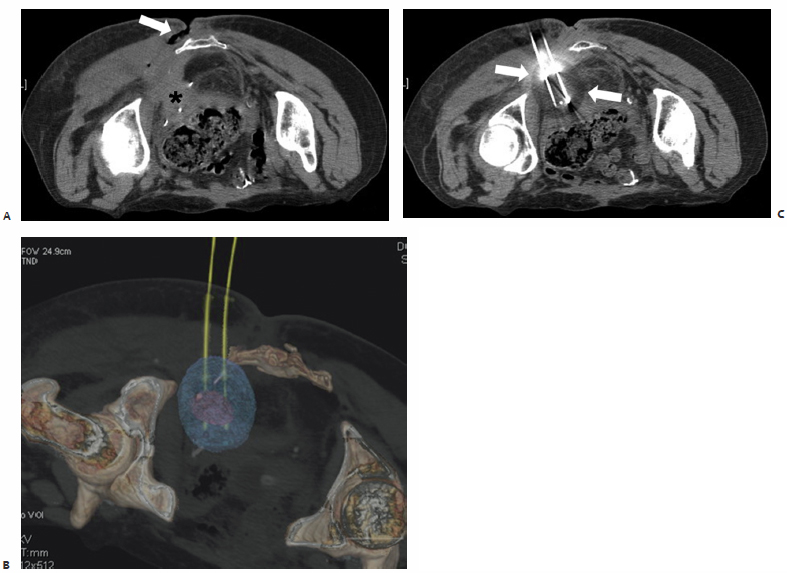
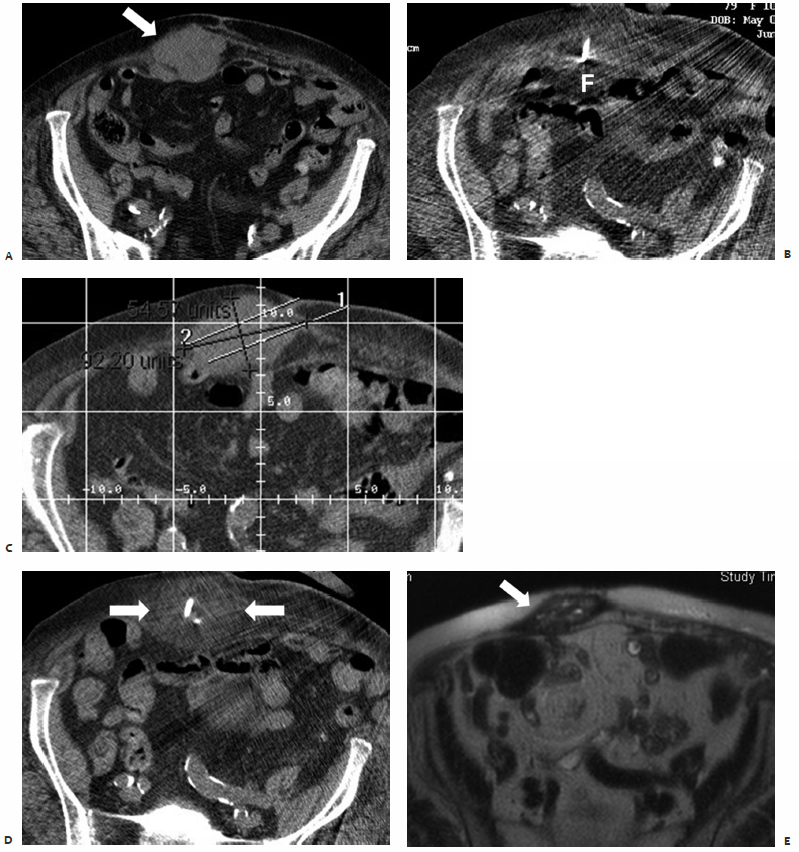
Fig. 9.4 A 65-year-old woman with colorectal carcinoma who had undergone surgical resection, chemotherapy, and external beam radiation with recurrent pelvic disease requiring permanent ileostomy and additional chemotherapy now presents with a second pelvic recurrence. (A) Attempted surgical resection of this left posterior pelvic mass (asterisk), biopsy proven metastatic colorectal carcinoma, with intraoperative radiation was complicated by infection, resulting in a persistent open, draining wound at the base of the spine posteriorly (arrow). The patient was deemed a suitable candidate for palliative percutaneous cryoablation of this left posterior pelvic sciatic notch mass prior to the initiation of further chemotherapy. Prophylactic antibiotics were prescribed to start 3 days before ablation. Intraprocedurally, additional IV antibiotics were administered, a Foley catheter was placed for decompression, and with the patient in the prone position, two 17-cm cryoprobes were placed under CT-fluoroscopic guidance into this 2.8 × 2.9–cm soft tissue mass (purple spherical mass in C) located adjacent to the sacrotuberous ligament. A 10-minute freeze and an 8-minute thaw were followed by a second 10-minute freeze. CT images obtained after the second freeze demonstrate formation of a 5 × 4 × 4–cm ice ball (B, arrows; C, blue sphere ) emanating from the cryoprobes. Sensory and motor function of the left lower extremity was intermittently examined and was without deficit. The Foley catheter was removed postprocedurally, and the patient was discharged within 4 hours without complication.
♦ Should I Ablate This? A Rational Approach
Essential questions to ask when deciding whether or not a given lesion is suitable for ablation are illustrated by examining the role of percutaneous ablation in the treatment of breast cancer.
What Is the Natural History of the Lesion? Current Standard of Care?
With a highly malignant natural history, breast cancer is known to spread progressively throughout surrounding breast parenchyma into surrounding fat and to overlying skin if left untreated. Metastatic disease is first found within the axilla and then more distantly in the lungs, liver, and skeleton due to hematogenous dissemination.
As evidence emerged in the early 1990s showing that mastectomy offered no survival advantage over breast-conserving therapies, the surgical management of early-stage breast cancer, tumor stages up to T2, has since progressed from radical mastectomy to modified radical mastectomy and then to breast conserving lumpectomy with or without postoperative radiation.66–69 Simultaneously, in patients with node negative disease, axillary lymph node dissection has been replaced by less invasive sentinel lymph node mapping and biopsy as a similar trend is affecting surgical management of the axilla.70
Patients with more advanced stages of disease commonly undergo treatment with neoadjuvant chemotherapy, with the intention of tumor down-staging so that a breast-conserving operation may be performed. Compounding this trend is the growing use of screening mammography and resultant increased early diagnosis of small lesions, even <1 cm, with the propensity for high long-term disease-free survival rates in these patients.71 Presently, T1a stage breast cancers are associated with a 90%, 20-year disease-free survival rate.69 Concern remains that present breast-conserving lumpectomy or segmental mastectomy may be overly deforming for such early-stage lesions.72
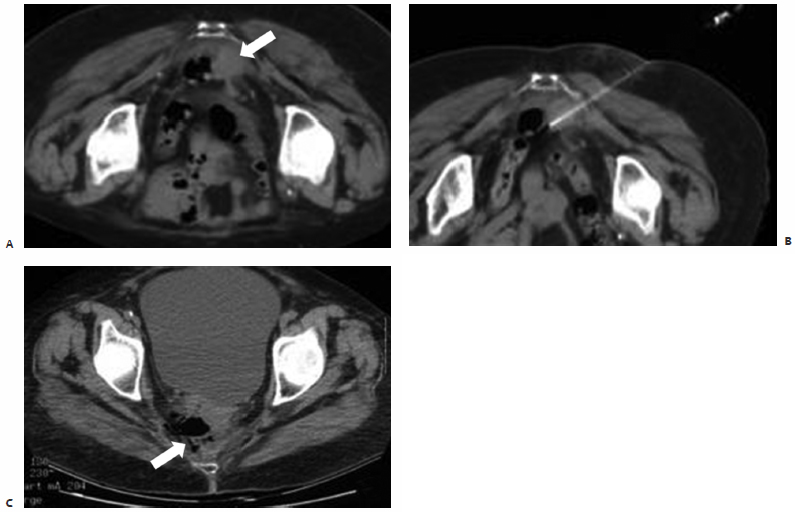
Fig. 9.5 A 77-year-old woman with a history of rectal carcinoma status post–resection, external beam radiation, and chemotherapy presents with a 2 × 3–cm round, soft tissue mass adherent to the sacrum and rectum deep within the pelvis. The patient complained of pelvic pain secondary to direct extension of this biopsy-proven focus of recurrent disease into the lumbosacral plexus. (A) CT scan prior to treatment showing the soft tissue mass (arrow). (B) Intraprocedurally, with the patient under conscious sedation, a Cool-Tip 3-cm active-tip RF electrode (Valley Laboratory, Boulder, CO) was percutaneously placed into the center of the lesion under CT-fluoroscopic guidance. A single 12-minute application was performed, and the patient was discharged postprocedurally within 3 hours. The patient presented 6 days after ablation with complaints of malodorous rectal discharge and increased right-sided pelvic pain. Plain films demonstrated an air-containing collection within the right hemipelvis. CT confirmed the presence of a 5 × 3 × 4–cm perirectal abscess anterior to the sacrum at the level of the sciatic notch within the ablation zone. (C) As the abscess was shown to directly communicate with the rectum (arrow), a confined perforation below the peritoneal reflection was confirmed on physical examination, for which the patient was started on antibiotics and instructed to follow a low roughage diet.
What Advantages Does Percutaneous Ablation Offer?
As a minimally invasive procedure, percutaneous ablation provides in situ treatment, likely reducing or eliminating the cosmetic defects and scarring associated with surgical excision. Operating room staffing and time, anesthesia needs, postprocedural recovery, complication rates, and associated costs would all be greatly decreased.73 Fat necrosis induced by thermal ablation may result in a slight change in focal fullness of the breast, but without en bloc removal of breast tissue nor the creation of a surgical incision, the two factors most related to the cosmetic outcome of breast-conserving surgery, percutaneous ablation may be a most desirable alternative therapy if local control rates comparable to those for surgical resection are able to be demonstrated.
The percutaneous nature of thermal ablation also makes it an ideal treatment option for nonsurgical candidates due to medical comorbidities or advanced staging. Elderly patients with limited life expectancy due to concurrent medical diagnoses, who are diagnosed with a small lesion, may benefit from this less invasive treatment option.
What Has Been Shown by Preliminary Clinical Studies? Animal Models? Ablate and Resect Trials?
Studies documenting the percutaneous use of RFA in the treatment of breast cancer consist of a relatively small number of patients and focus primarily on T1 disease.74 Patients with evidence of extensive intraductal disease or invasive lobular carcinoma are not ideal candidates for breast RFA, as localized discretely visualized lesions are best treated. Despite reports of ablation zones up to 4.5 cm in diameter, most treated lesions in RFA literature are ≤3 cm, as an additional 0.5- to 1.0-cm margin of surrounding normal breast tissue should ideally be ablated as well, the equivalent of an oncologic resection margin.75–79 Of note, patients status post–neoadjuvant chemotherapy were found not to be ideal candidates for image-guided percutaneous ablation due to the presence of imaging occult lesions on both mammography and US.80
Ablate and resect studies, including those by Jeffrey et al,81 Izzo et al,76 and Noguchi et al,82 utilize reduced nicotinamide adenine dinucleotide (NADH)-diaphorase staining to determine postablative cellular viability and report complete cell death in most if not all treated lesions. With evidence that immediate resection of ablated lesions was associated with an underestimation of induced cellular death, protocols subsequently called for delayed postablation resection. Burak et al78 excised ablated lesions at 1 to 3 weeks, and on pathologic exam no viable tumor was identified in 90% of patients. In clinical correlation, postablation follow-up MRI studies revealed well-defined ablation zones with no evidence of residual or recurrent disease.
Lumpectomy with postoperative radiation accounts for the majority of breast-conserving surgery currently being performed. The surgical literature documents that at least 75% of recurrent disease occurs at a site of prior lumpectomy, with positive margins found in 20 to 55% of resected specimens on pathologic examination.83 In an effort to combine the effectiveness of lumpectomy and cytotoxic effects of RFA, excision followed by RFA (eRFA), is a technique designed to ablate resection cavity margins at the time of initial operation. In a pilot study, 41 patients underwent lumpectomy followed by intraoperative RFA, essentially eRFA. Eleven of 41 specimens were found to have inadequate margins, only one of which was grossly positive requiring reexcision. Seventeen patients received postoperative radiation, and during a 24-month follow-up period, there were no reported local recurrences.84
What Is the Most Suitable Ablative Modality?
Clinical trials of cryoablation in the treatment of small invasive breast cancers have demonstrated varying efficacy.73 All lesions <1.0 cm and those between 1.0 to 1.5 cm with nonlobular histology nor extensive intraductal components were shown by Sabel et al73 to be successfully ablated with no pathologic evidence of viability. Therapy is limited to such small lesions, as the maximum achievable ice-ball diameter within the breast remains at 4.0 cm.
Cryoablation is presently Food and Drug Administration (FDA)-approved for the treatment of core biopsy–proven fibroadenomas, and is safely and effectively used in an outpatient setting requiring only local anesthesia. Patient satisfaction is rated at 88 to 97% postablation.85 Kaufman et al86 reported a mean volume reduction of 99% observed on US following cryoablation of 37 fibroadenomas, 84% of which were palpable, ranging in size from 0.8 to 4.2 cm (mean 2.1 cm).
Cryoablation has also been approved for use in localization of breast cancers in a procedure known as cryoprobe-assisted lumpectomy (CAL). A cryoprobe is percutaneously placed under US guidance into the center of the target lesion, and an ice ball is created with margins extending at least 5 mm circumferentially around the tumor. When compared with needle-wire localization in the treatment of invasive ductal carcinomas <1.7 cm, CAL was associated with a significantly improved rate of negative resection margins, a decreased volume of resected tissue, as well as improved cosmesis and shorter procedural times.87
What Is the Most Suitable Form of Intraprocedural Image Guidance? Is the Lesion Percutaneously Accessible? What Type of Sedation Is Necessary?
Radiofrequency ablation of the breast is most commonly performed under US guidance (Fig. 9.6), with occasional poorly visualized lesions requiring stereotactic treatment. Despite adequate visualization of electrode placement, the use of real-time intraprocedural US does not allow for clear visualization and monitoring of the developing ablation zone. As RF energy is deposited intralesionally, a hyperechoic focus develops, but posterior acoustic shadowing obscures visualization of the treatment zone in its entirety.76,78,79 With knowledge that ablated tumors show a reduction in peripheral vascularity, few groups have attempted to monitor ablation zone development by means of Doppler US.74 Cryoablation of breast cancers is also most commonly performed under US guidance.74 Despite real-time visualization of ice-ball formation and enlargement, deep margins of the lesion and the developing anechoic ablation zone are similarly obscured due to posterior acoustic shadowing secondary to ice-ball formation.
When using either RF or cryoablative technology, target lesions within the breast are percutaneously accessible. However, improper electrode/probe placement or underestimation of lesion size due to imaging techniques have led to incomplete ablation of target lesions.78,79 The success of breast ablation depends on accurate imaging of the lesion, as the appearance of a lesion on US may not correlate exactly with the pathologic extent of disease. This is the precise reason why ductal carcinoma in situ remains a challenge for percutaneous ablation, with even MRI known to underestimate the extent of disease. Incomplete ablation has also been found to result from lesion location. When lesions are excessively superficial or deep, <1 cm from the skin or chest wall, the risk of thermal burn injury to the skin or patient discomfort limits electrode/probe placement and achievement of cytotoxic temperatures.77
To date, the majority of reported studies document breast ablation performed in patients under general endotracheal anesthesia prior to surgical resection of the treated lesion. However, it seems logical that based on the tolerability of percutaneous ablation in a variety of systemic organs and the anesthetic properties of the freezing temperatures used in cryoablation, breast ablation should be similarly amenable to performance under conscious sedation.
What Do Clinical Safety and Long-Term efficacy Studies Show?
The most commonly reported complications of breast ablation include minor skin burns and minimal bruising.74 Within the first week following RFA, only approximately 13% of patients were noted to require acetaminophen with codeine for pain relief, and 95% of patients were amenable to repeat ablation if necessary.79 When using cryoablation, skin injury due to ice-ball formation remains a concern and may be prevented by precautionary measures such as the injection of warmed saline into subcutaneous tissues or topical application of warmed gauze pads.73
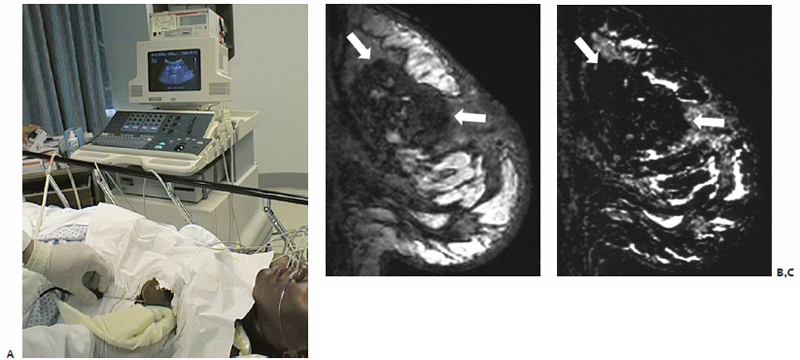
Fig. 9.6 (A) Percutaneous US-guided RFA of a left breast lesion. Sagittal postgadolinium T1-weighted (B) and subtraction (C) images of the left breast showing postablation changes (arrows), consistent with thermally induced necrosis.
As a safe minimally invasive treatment option with likely improved cosmetic outcomes, percutaneous thermal ablation has the potential to become an ideal treatment option for patients with breast cancer. However, prospective clinical trials are needed to determine the efficacy of ablation as a stand-alone treatment without surgical resection before nonoperative ablative management may be offered as a curative treatment.72 Long-term follow-up data must be collected and analyzed to establish efficacy comparable to surgical resection.
♦ Recipes for Success: Difficult Cases
Case 1: Abdominal Wall Metastasis
A 78-year-old woman with a history of myelodysplastic syndrome, congestive heart failure, chronic renal insufficiency, hypertension, chronic pulmonary effusions, osteoarthritis, anemia, and ovarian cancer status post–bilateral salpingo-oophorectomy with total hysterectomy and chemotherapy 9 years ago, presented with increasing Ca-125 laboratory values and a palpable mass in her right lower quadrant. A 5.5 × 4.0–cm lobulated mass contained within the right rectus sheath at the level of the umbilicus was identified on CT scan (Fig. 9.7). Subsequent MRI confirmed the presence of this lesion as well as a 2.7-cm mass with abnormal enhancement in the left kidney.
Within 2 weeks, the patient underwent percutaneous biopsy of the right anterior abdominal wall mass as well as biopsy and cryoablation of the now 3.0-cm solid mass in the medial aspect of the left kidney in close proximity to the ureter. Pathology specimens were consistent with poorly differentiated ovarian carcinoma metastatic to the abdominal wall, and primary renal cell carcinoma, respectively. Two-week follow-up CT scan revealed enlargement of the rectus sheath mass to 6.0 × 4.5 cm and stable postablation changes within the left kidney with no sign of residual disease.
Treatment tip: Cryoablation was chosen for use in this case due to the medial location of the lesion and adjacent ureter. When compared with RFA, cryoablative technology is associated with a markedly lower incidence of thermally induced ureteral strictures.
One month from the date of initial ablation, the patient underwent percutaneous cryoablation of the abdominal wall metastasis. Hydrodissection was used intraprocedurally to displace the large bowel away from the anticipated ablation zone. Four cryoablation probes were placed under CT-fluoroscopic guidance, and with the patient under conscious sedation two freeze–thaw cycles were performed (an 8-minute freeze followed by a 6-minute helium-induced active thaw). The patient tolerated the procedure well and was discharged within 2 hours of completion without complication.
At 1-week clinical follow-up, the patient denied any discomfort or systemic symptoms. Two-year MRI follow-up examination revealed stable postablation zones within both the left kidney and right rectus abdominus muscle, with no signs of residual nor recurrent disease, with a thermal scar at the latter site measuring only 4.1 × 1.8 cm.
Fig. 9.7 (A) CT scan showing the 5.5 × 4.0–cm lobulated mass contained within the right rectus sheath at the level of the umbilicus (white arrow). (B) Intraprocedurally, prior to the placement of cryoprobes, hydrodissection was used to the large bowel. A total of 200 cc of saline (fluid, F) was injected via a catheter percutaneously placed under CT-fluoroscopic guidance. Immediately after ablation with two cryoprobes (C), a screen-captured image (D) demonstrates the ice ball (arrows) resulting from two freeze–thaw cycles sufficiently encompassing the entirety of the lesion. (E) Two-year follow-up MRI demonstrates a stable postablation zone (arrow) with no evidence of active disease.
Treatment tip: Real-time intraprocedural visualization of the developing ice-ball on CT-fluoroscopy served as an additional safety measure preventing extension of the ablation zone into adjacent organs.
Treatment tip: In similar cases in which the target lesion not only abuts intraperitoneal structures but also is relatively superficial, there is a risk of injury to overlying skin. Air insuffation, delivery of air percutaneously via syringe and catheter, may be used to elevate the skin and subcutaneous tissues superiorly.
Case 2: Solitary Fibrous Tumor of the Buccal Space
A 64-year-old woman status post–excisional biopsy of a left buccal mass via transoral approach 1 year ago presented with complaints of facial asymmetry with left-sided swelling.88 Pathologic examination revealed a solitary fibrous tumor of the buccal space, a rare spindle cell neoplasm usually found within the thorax. The patient did not complain of facial numbness or paralysis resulting from this firm, mobile, nontender mass within the left malar soft tissues.
Having refused surgical reexcision due to the risk of facial nerve injury with resultant paralysis and incisional scarring, the patient was referred to the ablation clinic and elected to undergo percutaneous cryoablation. A well-circumscribed solid mass measuring 2.6 × 3.2 × 1.7 cm, distinct from the masseter muscle but displacing the parotid duct and facial vein medially, was seen on MRI. CT scan confirmed a lack of bony destruction of the nearby mandible and maxilla.
With the patient in the supine position, two argon-based 3-cm active-tip cryoprobes in parallel to one another spaced 1 cm apart were percutaneously placed into the lesion under CT-fluoroscopic guidance via a left transmalar approach. A total of four freeze–thaw cycles were performed, with the probes repositioned after the first two applications. Both local anesthesia and conscious sedation were used for patient comfort. Immediate postprocedure CT revealed a 3.3 × 4.5 × 3.0–cm freeze zone encompassing the lesion and a 5-mm circumferential margin. The patient tolerated the procedure well, and was discharged home within 2 hours of completion without complication.
At 2 days postablation, swelling of the malar soft tissues, sloughing of the buccal mucosa, and a small eschar on the skin surface superficial to the treatment zone were noted. These findings gradually resolved, and at 6-month clinical follow-up the patient noted no residual symptoms or signs of sensory or motor nerve injury. MRI at this time revealed an organized 2-cm fluid collection within the buccal soft tissues with a small area of peripheral enhancement. US-guided FNA biopsy revealed necrotic cells with no evidence of recurrence. By 14 months, the treatment cavity showed near-complete collapse on MRI, with only minimal peripheral posterior enhancement, and the patient’s facial swelling had completely resolved.88
Treatment tip: With a 3-cm active-tip, each 1.7-mm cryo-probe is able to create a −20°C isotherm or freeze-zone 4.2 × 2.1 cm, containing a central −40°C isotherm 3.5 × 1.4 cm. In this case, the size of the target lesion necessitated the use of two cryoprobes in parallel to generate a treatment zone large enough to encompass both the lesion and an oncologic margin. The reported freeze zone measured 3.3 × 4.5 × 3.0 cm.88
Treatment tip: Cryoablation was the modality of choice for this case, as it is less disruptive to underlying supportive tissues and less likely to induce nerve injury, important features when working in delicate facial tissues in close proximity to the facial nerve.
Case 3: Recurrent Pediatric Malignancies
A 9-year-old girl diagnosed with Ewing sarcoma of the pelvis at age 5 for which she received chemotherapy and radiation presented with clinical recurrence in the left temporal calvarium and right ischium and acetabulum, treated with a combination of surgical debulking, chemotherapy, and additional radiation therapy over the past several years.89 She then presented with right thigh and buttock swelling with pain radiating to the perineum and a nonfocal neurologic examination.
An MRI demonstrated a new left sacral mass with significant epidural extension into the spinal canal and secondary compression of the thecal sac and nerve roots. Repeat radiation was deemed inappropriate due to prior radiotherapy to this area. Given the superficial location of the lesion and involvement of sacral nerve roots, percutaneous cryoablation was deemed the most appropriate means by which to provide pain relief, control tumor extension, and preserve neurologic function.
Under general anesthesia, argon-based cryoablation was performed with intraprocedural monitoring of somatosensory-evoked potentials (SSEPs) of the L5, S1, and S2 territories at 2-minute intervals to prevent nerve injury. Five 3-cm active-tip cryoprobes were used, delivering two freeze–thaw cycles (10-minute freeze and 8-minute thaw). Postprocedural neurologic examination was without change, and within 24 hours the patient was pain free, no longer requiring twice daily morphine.
Within 48 hours of ablation, the patient complained of constipation and an inability to urinate. After 3 months, the constipation resolved but the patient remained incontinent of bladder and bowel function, requiring long-term intermittent catheterization. Over the next several months, the patient did not require narcotic pain medication, and after enjoying an active summer returned to school in the fall.89
Treatment tip: Warm balloons filled with sterile water were placed on the patient’s skin at the site of percutaneous cryoprobe entry to prevent dermatologic injury.
Treatment Tip: The use of SSEPs allowed for safe treatment in close proximity to the S1 nerve. Thus, left lower extremity function was not compromised.
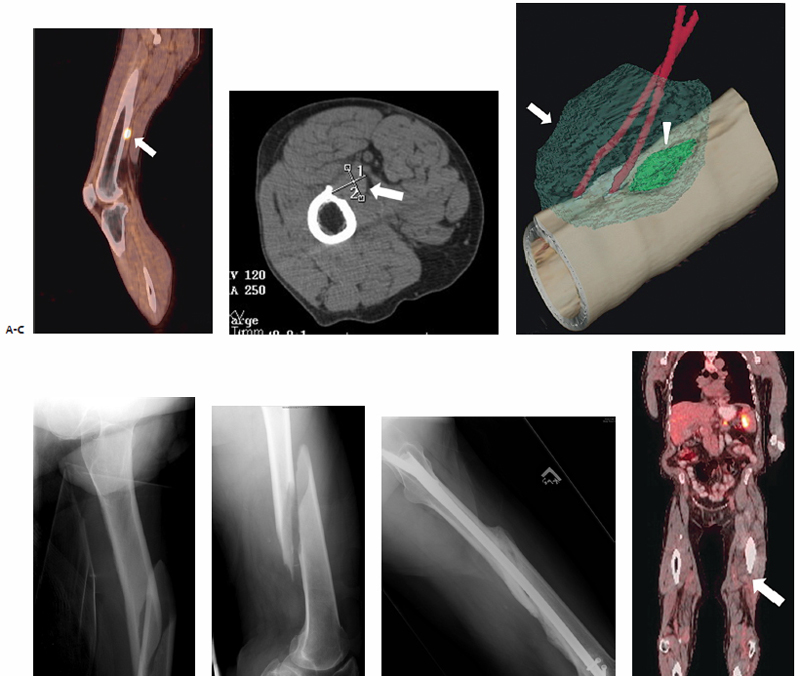
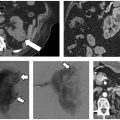
Fig. 9.8 (A) Sagittal PET-CT image of the left lower extremity shows an intense focus of FDG accumulation corresponding to a metastatic lesion involving the posterior femoral cortex (arrow). (B) Axial CT demonstrates a fusiform 2.6 × 1.0­–cm mass scalloping the posterior distal portion of the left femoral cortex (arrow). (C) Intraprocedurally, two cryoprobes were percutaneously placed in the craniocaudal plane, and two freeze–thaw cycles were performed. The resultant ice ball (arrow) is shown to encompass the entirety of the target lesion (arrowhead). (D–F) Frontal and lateral radiographs of the left femur obtained 5 weeks postablation after the patient sustained a fall demonstrate an oblique fracture with anterior displacement of the distal femoral diaphysis (F), which required internal fixation. (G) Two-year postablation follow-up PET-CT demonstrates no signs of active FDG-avid disease within the ablation zone (arrow).
Case 4: Recurrent Pediatric Malignancies
A 7-year-old girl status post–multiagent chemotherapy, surgical resection, and radiotherapy for anaplastic stage III Wilms’ tumor diagnosed at the age of 3 presented with abdominal pain and jaundice.90 Local recurrence within the resection bed was confirmed on laparoscopic biopsy, prompting the initiation of high-dose chemotherapy, further surgical resection, and combined brachytherapy and external beam radiation. However, 2 years later, at the age of 11, the patient developed a second recurrence within the renal fossa, which despite chemotherapy continued to increase in size, eventually abutting the celiac plexus, necessitating daily fentanyl and hydromorphone use for pain control.
Combined RFA and brachytherapy were to be performed with the intent of both pain reduction and local tumor control. With the patient under general anesthesia, a single 2-cm active-tip RF electrode was placed intralesionally in the left paraspinal region under CT-fluoroscopic guidance. After two RF applications, the intratumoral temperature was recorded at 77.5°C. A 6-French brachytherapy catheter was then percutaneously placed to deliver a single fraction of 1500 cGy with high-dose rate iridium (192IR).
Despite an increased analgesic requirement in the first few postprocedural days, within 1 month the patient no longer required IV narcotics, and her pain-control regimen was reduced to only a low-dose transdermal patch and an intermittent moderate-dose oral narcotic. Eight-month follow-up CT scan showed a stable postablation zone with no evidence of recurrence. This relatively minimal analgesic regimen remained sufficient until she died of cardiac arrest in the setting of refractory pancytopenia and fever due to overt leukemia at 9½ months postablation.90
Treatment tip: The cytoreductive effects of RFA may increase the degree of local tumor control achievable with radiation therapy. Cellular response to heating at the margin of thermal injury results in an inflammatory infiltrate and the formation of granulation tissue, increasing locoregional vascularity and tissue oxygenation both acutely following ablation and during healing. Any residual tumor cells at the periphery of the ablation zone are thus in an oxygen-rich environment and are more susceptible to radiotherapy, which relies on oxygen in the production of lethal free radicals and superoxide anions. In the acute setting, hyperthermia and brachytherapy may also be synergistic.91,92
Case 5: Metastatic Melanoma
An 80-year-old man with widely metastatic melanoma presented with a fusiform 2.6 × 1.0–cm mass scalloping the posterior distal portion of the left femoral cortex (Fig. 9.8). The lesion was proven on biopsy to be a metastatic focus. The patient was amenable to prophylactic percutaneous cryoablation, precluding the need for surgical resection and internal fixation.
Intraprocedurally, two cryoprobes were percutaneously introduced into the target lesion in a craniocaudal orientation. After administration of conscious sedation, two freeze–thaw cycles (10-minute freeze with 8-minute thaw followed by 5-minute freeze for the first cycle and 10/8/10 for the second) were performed. Neurovascular examination at 5 minutes into the first freeze revealed decreased sensation with preservation of motor function in the left foot. An improvement in sensation was found upon reexamination at 5 minutes into the second cycle.
Upon discharge on the day of ablation, a mild loss of motor activity and weakness in the left lower extremity was noted, necessitating the use of a walker. In postablation follow-up 1 week later, the weakness had resolved despite residual decreased flexion of the left ankle. The patient was ambulating with a cane at this time and noted numbness and tingling on the plantar aspect of the left foot. Because of recent-onset swelling of the distal left lower extremity beginning at the level of the thigh, deep venous thrombosis was ruled out by US examination. Routine follow-up PET-CT imaging revealed decreased uptake within the ablation zone and no signs of residual or locally recurrent disease.
The patient fell 5 weeks after ablation, so frontal and lateral radiographs of the left hip and femur were obtained. An oblique fracture with approximately one shaft width anterior displacement of the distal femoral diaphysis was seen. This midshaft fracture extended through to the previously identified posterior cortical lucency, suggesting the presence of a postablative stress riser predisposing to pathologic fracture at this site.
Treatment tip: Cryoablation was selected for this case so as to reduce the risk of thermal injury to the tibial nerve and offer increased penetration of this bony lesion.
- Published data on ablating esoteric organs are lacking. The decision to ablate or not should depend on the specific risk/benefit ratio.
- The risks of ablating head and neck tumors are greater than the risks of ablating tumors elsewhere in the body; therefore, the expected benefit should also be greater.
- Patients planned for adrenal tumor ablation should be premedicated with alpha- and beta-blockers to prevent a hypertensive crisis or arrhythmia during ablation.
- Hypertension or arrhythmias usually occur during the thawing cycle of adrenal cryoablation.
- Percutaneous RFA is an effective minimally invasive alternative to surgical resection for a variety of benign and malignant soft tissue masses in the head and neck, including hyperfunctioning parathyroid adenomas, lingual thyroid, laryngeal papillomata, and buccal lymphatic malformations.48,52–54