Fig. 16.1
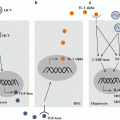
The altered peptide/major histocompatibility complex class I (pMHCI) repertoire caused by antigen-processing defect (APD). In ERAAP-deficient or tapasin-deficient cells, the total amount of pMHCI on the cell surface is reduced to 70–80 % and 10 % of wild type, respectively. Both of two APD conditions present quantitatively reduced pMHCI repertoire, however, the quality of presented peptides is largely altered from that of wild-type peptides. While canonical peptides are reduced, APD peptides which are never seen on wild type are preferentially presented in the absence of ERAAP or tapasin. APD peptides are immunogenic and able to elicit CD8+ T cell responses when immunized into wild-type animals.
The generation of APD peptides has been known in the absence of TAP, ERAAP, or tapasin function. Van Hall and colleagues demonstrated that TAP-deficient cells are immunogenic to wild-type mice, and identified the H-2Db-restricted antigenic peptide derived from the Lass5 gene, which encodes ER-membrane spanning protein (van Hall et al. 2006). Shastri and colleagues identified the another APD peptide encoded in Fam49b protein that is presented in the absence of ERAAP (Nagarajan et al. 2012). This particular peptide is of interesting because of its Qa-1b-restricted presentation. Qa-1b in mouse (HLA-E in humans), which belongs to non-classical MHCI family, is known to present non-polymorphic peptides derived from the signal sequence of classical MHCI and interacts with NKG2A/CD94 heterodimer on natural killer (NK) cells or a small subset of CD8+ T cells (Braud et al. 1998; Vance et al. 1998). This finding clearly indicates that the immune system takes advantage of non-classical MHCI for surveillance of APD peptide, along with the fact that Qa-1b is capable of presenting a diverse pMHCI repertoire (Oliveira et al. 2010). Recently, we have found that cells lacking tapasin also display the qualitatively altered pMHCI and are able to elicit new CD8+ T cell responses in immunized wild-type mice (Kanaseki et al. 2013). Tapasin is covalently bound to Erp57 and recruits an empty MHCI next to TAP, thus the absence of tapasin could represent the compromised condition of PLC formation in the cells. Angiotensin converting enzyme (ACE) is a carboxypeptidase that is a potential candidate of an another pMHCI editor, and, in fact, new CD8+ T cell responses specific to ACE-deficient cells are also generated (Shen et al. 2011). Rock and colleagues reported the altered pMHCI repertoire in the cells lacking three immunoproteasome catalytic subunits; however, new CD8+ T cell responses specific to it are not induced, indicating the distinct nature of APD in the cytoplasm (Kincaid et al. 2011).
16.3 Unique Peptide/MHC Class I (pMHCI) Repertoire Produced by APD
We next consider how the pMHCI repertoire is altered from the original repertoire in the cells with APD in the ER, particularly in the absence of ERAAP, or tapasin. The source peptides coming from the cytoplasm could be comparable among these two APD conditions and wild type without APD, contrary to the absence of TAP, in which the peptide supply is extremely limited and membrane proteins are preferentially processed. In addition, wild-type CD8+ T cells induced by immunizations against tapasin-deficient cells do not cross-respond to ERAAP-deficient cells, and vice versa, which suggests that the immunogenic repertoires of APD peptides produced in ERAAP- or tapasin-deficient cells are exclusive to each other. Therefore, the nature of the altered pMHCI repertoire reflects the roles of the defected molecule in MHCI antigen processing, and the immune system potentially distinguishes the cells with different APD conditions.
Both of the immunogenic pMHCIs presented on ERAAP- and tapasin-deficient cells are unstable, and the specific CD8+ T cell responses to them are readily diminished by Brefeldin A treatment. In addition, an immunization of ERAAP-deficient cells to wild-type mice elicits B cell responses which produce specific antibodies. Considering that, the unique pMHCIs formed with APD peptides are structurally distinguished from conventional ones (Hammer et al. 2007a). Furthermore, the large-scale peptide sequencing by the use of mass spectrometry has demonstrated that the H-2Db and H-2Kb peptides produced in the absence of ERAAP are unusually long on average, most likely due to the loss of their aminopeptidase activity to trim the N-terminal end of precursors (Blanchard et al. 2010). It is well characterized that unusually long peptides bound to MHCI molecule make a bulge at the center and often elicit strong CD8+ T cell responses (Tynan et al. 2005). Meanwhile, the APD peptides produced in the absence of tapasin frequently lack consensus anchor residues for binding to MHCI (Kanaseki et al. 2013). Their MHCI binding scores are low according to the prediction and indeed consistent with the proposed role of tapasin in selecting high-affinity peptides. The crystal structure of tapasin–Erp57 complex in the PLC estimates that tapasin interacts with the mobile α2-1 segment of MHCI, that is, interaction with tapasin potentially influences the shape of peptide-binding groove (Lewis and Elliott 1998; Dong et al. 2009). Thus, the unusual anchorless pMHCI which were supposed to be retained inside the ER are unexpectedly sorted and displayed on the surface in the absence of tapasin. The molecular mechanisms by which APD peptide are preferentially processed in the absence of tapasin should be further investigated.
16.4 APD and Immunotherapy Against Tumors
ERAAP and tapasin are usually expressed in eukaryotic cells expressing MHC I on the cell surface, therefore most of normal cells do not present APD peptides and they are not targeted by specific CTLs induced by APD. However, the loss of ERAAP, and in particular, tapasin expression has been reported in many malignant tumors including lung cancers, colorectal cancers, and head and neck cancers (Ogino et al. 2006; Jiang et al. 2010; Lou et al. 2005; Atkins et al. 2004). These findings suggest that not a few tumors are considered to be the heterogeneous mixture consisting of tapasin-deficient variants. Precise molecular mechanisms by which tapasin expression is down-regulated in tumors are under debate for now, but a fact is that tapasin-deficient variants of tumors grow up in vivo despite of the very low level of MHCI expression which makes cells susceptible to NK cell lysis. Moreover, a positive correlation between the loss of tapasin expression and poorer prognosis has been reported. As is expected, CTLs targeting a conventional peptide are able to lyse human tumor cells that express tapasin, however, CTLs no longer lyse the tapasin-deficient variant of that tumor cells, even when the antigen is expressed within (Kanaseki et al. 2013). Such tumor cells lacking tapasin expression is an immune escape variant of that tumor and could become a serious issue in CTL immunotherapy. Because CTLs induced by APD distinguish tapasin-deficient tumor cells from tapasin-proficient normal cells and are able to lyse them in vivo, we propose to apply the CTL responses induced by APD peptides for targeting tumor cells. In mouse case, it has been shown that CTL responses to the peptide derived from lass5 gene mentioned above successfully lysed TAP-deficient variant of tumor cells. Exploiting APD peptides and a new mode of CTL induction is definitely a candidate solution against the immune-escape variants of tumors.
References
Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Storkel S, Seliger B (2004) MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer 109(2):265–273
Belicha-Villanueva A, Golding M, McEvoy S, Sarvaiya N, Cresswell P, Gollnick SO, Bangia N (2010) Identification of an alternate splice form of tapasin in human melanoma. Hum Immunol 71(10):1018–1026. doi:10.1016/j.humimm.2010.05.019
Blanchard N, Kanaseki T, Escobar H, Delebecque F, Nagarajan NA, Reyes-Vargas E, Crockett DK, Raulet DH, Delgado JC, Shastri N (2010) Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. J Immunol 184(6):3033–3042. doi:10.4049/jimmunol.0903712 CrossRefPubMedCentralPubMed
Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ (1998) HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391(6669):795–799. doi:10.1038/35869 CrossRefPubMed
Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM (2009) Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity 30(1):21–32. doi:10.1016/j.immuni.2008.10.018 CrossRefPubMedCentralPubMed
Elliott T, Williams A (2005) The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol Rev 207:89–99. doi:10.1111/j.0105-2896.2005.00311.x CrossRefPubMed
Fruci D, Ferracuti S, Limongi MZ, Cunsolo V, Giorda E, Fraioli R, Sibilio L, Carroll O, Hattori A, van Endert PM, Giacomini P (2006) Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol 176(8):4869–4879CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree