are evaluated with respect to both accuracy and reproducibility. Accuracy is the difference between the measured value and the true value, which implies that a true value is known. Clearly, this may present difficulties when dealing with biologic specimens. The National Committee for Clinical Laboratory Standards (NCCLS) and the Clinical and Laboratory Standards Institute (CLSI) have attempted to develop standards to assess the accuracy of blood smear examination11 and automated blood cell analyzers.2 Automated instrumentation requires regular quality assurance evaluations and careful calibration to reach expected performance goals and the ability to collect accurate and reproducible data.2,12,13 In addition, the International Consensus Group for Hematology Review has suggested criteria that should lead to manual review of a specimen after automated analysis and differential counting.3
TABLE 1.1 REPRODUCIBILITY OF BLOOD COUNTING PROCEDURES | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
and use a very small sample ranging from 35 to 150 µl for a full CBC analysis. Using flow cytometric technologies, some analyzers also have the ability to detect specific blood cell populations by specific antigen expression, such as detection of CD34 peripheral blood stem cells or leukemic blasts.24,25 and 26 Integration of data from cytochemical or antigenic staining and light scatter properties has improved the accuracy of the five-part differential and decreased the numbers of unidentifiable cells requiring technician review for identification.
in manual Hct determinations arises from trapping of plasma in the red cell column. This may account for 1% to 3% of the volume in microcapillary tube methods, with macrotube methods trapping relatively more plasma.33,34 It should be noted that abnormal red cells (e.g., sickle cells, microcytic cells, macrocytic cells, or spherocytes) often trap higher volumes of plasma due to increased cellular rigidity, possibly accounting for up to 6% of the red cell volume.34 Very high Hcts, as in polycythemia, may also have excess plasma trapping. Manual Hct methods typically have a precision coefficient of variation (CV) of approximately 2%.33
TABLE 1.2 REPRODUCIBILITY OF RED CELL INDICES | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
abnormal earlier in nutritional deficiency anemias than other red cell parameters, especially in cases of iron deficiency anemia.43,53 RDW is particularly useful in characterizing microcytic anemia, allowing discrimination between uncomplicated iron deficiency anemia (high RDW, normal to low MCV) and uncomplicated heterozygous thalassemia (normal RDW, low MCV),43,53,54 and 55 although other tests are usually required to confirm the diagnosis.56 RDW is also useful in identifying red cell fragmentation, agglutination, or dimorphic cell populations (including patients who have had transfusions, have sideroblastic anemias, or have been recently treated for a nutritional deficiency).53,57
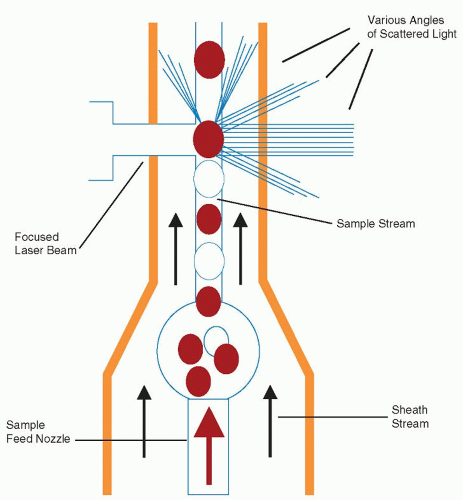
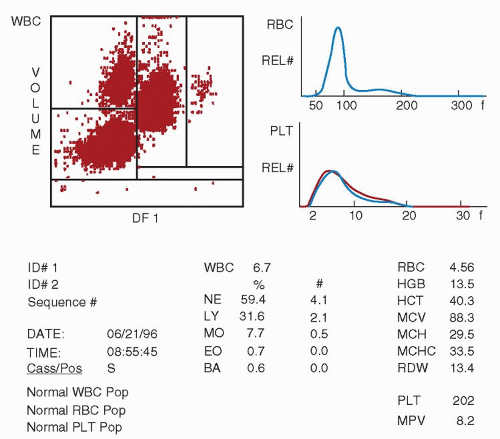
lymphocyte nuclei. Abnormal cell populations will generate a flag, indicating a need for morphologic review of the peripheral smear.3 Analysis using this technique examines thousands of cells per sample, increasing statistical accuracy.16
MCHC calculations.40 Older analyzers reported spurious increases in hemoglobin levels when white cell counts exceeded 30 × 109/L due to increased turbidity, but this is decreased with newer flow systems so that hemoglobin levels remain extremely accurate in the face of white blood cell counts as high as 100 × 109/L.16 Extremely high white cell counts may also falsely raise the red cell count and Hct as the white cell count is incorporated into the red cell count. High glucose levels (>400 to 600 mg/dl) and the associated hyperosmolarity cause red cell swelling and generate a high MCV and Hct with a falsely low MCHC.36,106 Increased turbidity associated with hyperlipidemia may also cause falsely elevated hemoglobin determinations, MCH, and MCHC.41
TABLE 1.3 DISORDERS AND CONDITIONS THAT MAY REDUCE THE ACCURACY OF BLOOD CELL COUNTINGa | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree