FIGURE 18-1. Selective antiestrogen treatment inhibits pituitary tumor growth in vivo. Pretreatment (baseline) and posttreatment tumor volumes and serum prolactin (PRL) levels after mini osmotic pumps infusion of vehicle or antiestrogen ICI-182780 (0.5 µg/day) was infused in 20 female Wistar-Furth rats harboring subcutaneous pituitary tumors. All animals developed tumors. Each bar represents mean ± SEM for 10 animals per group. *P = 0.03; **P < 0.001.
(From Heaney AP, Fernando M, Melmed S: Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest 109:277–283, 2002.)
Studies on the microvascular density of prolactinomas show conflicting results: One study by electronic microscopy did not disclose differences between vascular density of the normal pituitary and microprolactinomas, but macroprolactinomas exhibited a much lower degree of vascularization. A more recent study using immunohistochemistry with antibodies for different endothelial markers observed that microprolactinomas are less vascular than macroprolactinomas. Additionally, microvascular density was related to tumor invasiveness and malignancy.22
The decrease of dopaminergic inhibition seems to play a role, at least a permissive one, in prolactinoma development. Lesions in the tuberoinfundibular dopaminergic neurons in female rats bearing prolactinomas were described.23 Several studies have shown the development of prolactinomas in mice with D2 receptor knockout, with this phenotype being more severe and presenting a faster evolution in females and in animals treated with estrogens.24,25 To date, no “natural” mutations were found in the D2 receptor gene.26 PRLr(−/−) mice exhibited more intense hyperprolactinemia and larger tumors than did age-matched Drd2(−/−) mice, and there were cumulative effects in compound homozygous mutant male mice. This fact suggests that PRL inhibits lactotrophs not only by the activation of hypothalamic dopamine neurons, but also directly within the pituitary in a dopamine-independent fashion.
Prolactinomas associated with MEN1 present inactivating mutations characterized by loss of heterozygosity in locus 11q13 and mutations in the menin-codifying gene. They tend to be larger and more aggressive than their sporadic counterparts.12 Sporadic prolactinomas may exhibit loss of heterozygosity but without mutations in the menin gene detected to date, suggesting the presence of a tumor suppressor gene located within chromosome 11q13 yet distinct from MEN1.27 The recently described inactivating mutations of the gene encoding aryl hydrocarbon receptor–interacting protein (AIP) on chromosome 11q13.3 are frequently found in isolated familial pituitary adenomas (mainly somatotropinomas but also prolactinomas)28 but rarely in sporadic pituitary tumors.29 Finally, mutations in the proto-oncogene ras and in the tumor suppressor gene TP53 can be linked to the development of the rare PRL-secreting carcinomas.3
Pathology
The terms microadenoma and macroadenoma, coined by Hardy,30 represent pituitary adenomas measuring less or more than 1 cm, respectively. Microprolactinomas are found mainly in young women, usually located in the lateral portions of the pituitary. They are generally enclosed within a pseudocapsule but also can be invasive.31 Macroprolactinomas also can be enclosed but usually expand to the optic chiasmal region or invade local structures such as the cavernous sinus or the sellar floor and sphenoid sinus.31 Histologically, there are no differences between microprolactinomas and macroprolactinomas, which are usually “chromophobic” on hematoxylin-eosin staining. For this reason, many pituitary adenomas previously classified as “functionless” before the development of a radioimmunoassay for PRL were prolactinomas. This fact is explained by the use of electronic microscopy, which characterized most prolactinomas as sparsely granulated: oval and slightly irregular nuclei, complex rough endoplasmic reticulum, large Golgi complexes, and sparse spherical or pleomorphic secretory granules, measuring 130 to 500 nm (Fig. 18-2).32 The hallmark of these tumors is the so-called misplaced exocytosis, the extrusion of secretory granules along the lateral cell border. This phenomenon diagnoses PRL secretion either by normal or by neoplastic lactotrophs. The rare, densely granulated lactotroph adenoma is strongly acidophilic with strong, diffuse immunostaining for PRL in the cytoplasm. The rough endoplasmic reticulum is less prominent, and the secretory granules are bigger (500 to 700 nm) and more numerous than in its sparsely granulated counterpart.32
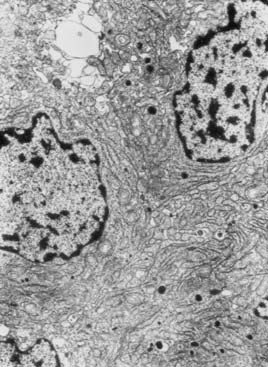
FIGURE 18-2. Electronic microscopy of a sparsely granulated prolactinoma, showing sparse secretory granules, well-developed rough endoplasmic reticulum, and prominent Golgi complexes.
Electron microscopy usually is not required for prolactinoma diagnosis since the immunohistochemical assessment of PRL production became routinely available. This technique directly characterizes PRL-secreting adenomas, ruling out “functionless” macroadenomas associated with hyperprolactinemia due to hypothalamus-pituitary disconnection, the so-called pseudoprolactinomas.33 A study of 120 unselected necropsies showed that 27% of them harbored pituitary microadenomas without clinical expression, 40% of which immunostained for PRL.11 These data indicate that there could be clinically and hormonally non–PRL-secreting pituitary adenomas that are immunohistochemically positive for this hormone. The significance of this finding for the natural history of prolactinomas is unknown.
ACIDOPHIL STEM CELL ADENOMA
Exceptionally hyperprolactinemic patients might exhibit mild or no clinical features of acromegaly associated with biochemical evidence of slight serum GH elevation. This situation is due to the presence of the rare and aggressive acidophil stem cell pituitary adenoma.34 The acidophilia is attributable to mitochondrial accumulation called oncocytic change. Immunohistochemical analysis is positive for PRL, and occasionally there is a scant positivity for GH. The definitive diagnosis requires electron microscopy, which depicts enlarged mitochondria. Scattered cells containing juxtanuclear fibrous bodies are similar to cells of sparsely granulated somatotroph adenomas. Misplaced exocytosis is present. The secretory granules are sparse and small, measuring 150 to 200 nm.
NONTUMORAL LESIONS ASSOCIATED WITH HYPERPROLACTINEMIA
Many nontumoral conditions can be mistaken for prolactinomas. Thyrotroph hyperplasia is associated with primary hypothyroidism due to loss of feedback (Fig. 18-3).35 The correct diagnosis is important because this condition regresses with thyroid hormone replacement.35 Idiopathic lactotroph hyperplasia is a rare cause of hyperprolactinemia that mistakenly can be taken for an expanding macroprolactinoma. The pituitary mass shape can help in the differential diagnosis. Inflammatory lesions such as lymphocytic hypophysitis (occurring mainly during pregnancy and puerperium) and sarcoidosis can be misdiagnosed as lactotroph adenoma.36,37
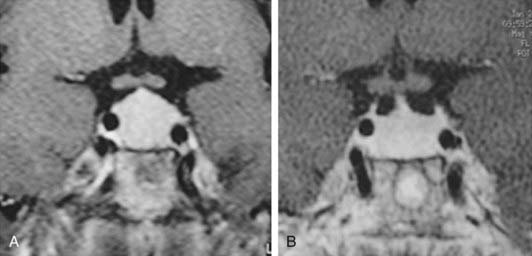
FIGURE 18-3. Magnetic resonance imaging (gadolinium-enhanced T1-weighted coronal views) of a 41-year-old woman with primary hypothyroidism before (A) and after 1 month on levothyroxine replacement therapy (B).
(From Bronstein MD: Problems in the differential diagnosis of the hyperprolactinemic patient. Clinical Endocrinology Update 2003, syllabus pp 241–247; with permission of the Endocrine Society.)
PITUITARY PROLACTIN-SECRETING CARCINOMAS
Pituitary PRL-secreting carcinomas are exceedingly rare tumors that exhibit morphologic features indistinguishable from those of PRL-secreting adenomas. In addition to local invasiveness, distant intracranial and extracranial metastases in the nervous system and visceral metastases are the clues for the diagnosis of malignancy. Regarding intracranial lesions, sometimes it is difficult to distinguish between contiguity of an invasive prolactinoma and true metastasis from a PRL-secreting carcinoma.3
Differential Diagnosis of Hyperprolactinemia
The main causes of hyperprolactinemia are listed in Table 18-1.
Table 18-1. Causes of Hyperprolactinemia
| PHYSIOLOGIC |
| DRUG-INDUCED |
| PATHOLOGIC |
PHYSIOLOGIC HYPERPROLACTINEMIA
Throughout pregnancy, the size of a normal pituitary increases up to 136%, according to magnetic resonance imaging (MRI) studies.38 This extensive growth is due to estrogen-induced hypertrophy and hyperplasia of lactotrophs, leading to progressive increase in PRL production and its hypersecretion during pregnancy.39 Placental estrogen production stimulates lactotroph mitosis, PRL mRNA levels, and PRL synthesis, leading to a stepwise increase in serum PRL levels, achieving mean levels of 200 ng/mL at the end of pregnancy and up to 450 ng/mL in some cases. Serum PRL levels decline quickly after delivery but are maintained slightly increased in nursing women several months, especially after breastfeeding. At birth, newborn serum PRL concentrations are elevated nearly 10-fold, probably as a result of the stimulatory effect of maternal estrogen levels.40
Because exercise and nonspecific stress are physiologic causes of hyperprolactinemia, there is a concern that the stress-induced PRL increase could lead to hormone elevation during venipuncture, and a period of rest before blood withdrawal is still recommended in many laboratories. A report by Vieira and coworkers,41 which included a large population, provided evidence that rest before blood collection may be needed in only a few patients.
PHARMACOLOGIC HYPERPROLACTINEMIA
Among medications that increase serum PRL, dopamine receptor blockers are the most potent. Neuroleptics (e.g., sulpiride, haloperidol, chlorpromazine, risperidone) and antiemetic drugs (e.g., metoclopramide, domperidone) can elevate serum PRL to levels that usually are detected with prolactinomas.42 Serotoninergic and antihistaminergic drugs are less potent than antidopaminergic medications. The calcium channel blocker verapamil elevates serum PRL levels probably by decreasing central dopamine generation, possibly through N-type calcium channels.43 It was shown that protease inhibitors used for treatment of acquired immunodeficiency syndrome can cause hyperprolactinemia, but the mechanism is unknown.44 A detailed inquiry about drug use is mandatory for all hyperprolactinemic patients.
PATHOLOGIC HYPERPROLACTINEMIA
Hyperprolactinemia is present in about 40% of acromegalic patients as a result of GH/PRL co-secretion by the same or by different tumor cells or secondary to hypothalamus-pituitary disconnection.45 Because of the characteristic features of acromegaly, the differential diagnosis is usually not a problem. As already mentioned, in patients harboring the rare acidophil stem cell pituitary adenoma, serum GH is usually low compared with PRL levels, however, and acromegalic features are usually absent or minimally expressed.34 In some cases, PRL resistance to dopamine agonists can be a clue for the differential diagnosis with prolactinomas.
Hyperprolactinemia secondary to impaired hypothalamic/tuberoinfundibular dopamine secretion or to stalk or even intrapituitary disconnection can be caused by tumors, inflammatory diseases, or trauma. In these cases, PRL is produced by normal lactotrophs and rarely exceeds 150 µg/L. The differential diagnosis of macroprolactinomas is mainly with clinically nonfunctioning pituitary adenomas (pseudoprolactinomas) and, to a lesser extent, with craniopharyngiomas.46 Other tumoral lesions, such as meningiomas and chordomas, and nontumoral conditions, such as “empty sella” syndrome and even intrasellar aneurysms, can be associated with hyperprolactinemia, however (Fig. 18-4).47 The differential diagnosis between macroprolactinomas and pseudoprolactinomas is crucial regarding their primary treatment—medical for macroprolactinomas and surgical for pseudoprolactinomas. Patients with nonfunctioning tumors treated with dopamine agonists were considered as having resistant macroprolactinomas, owing to the absence of tumor shrinkage even with the (obvious) PRL decrease to very low or undetectable levels (Fig. 18-5).48 A clue to differentiate pseudoprolactinomas from true prolactinomas is the dramatic early PRL decrease with bromocriptine doses of 1.25 mg/day.
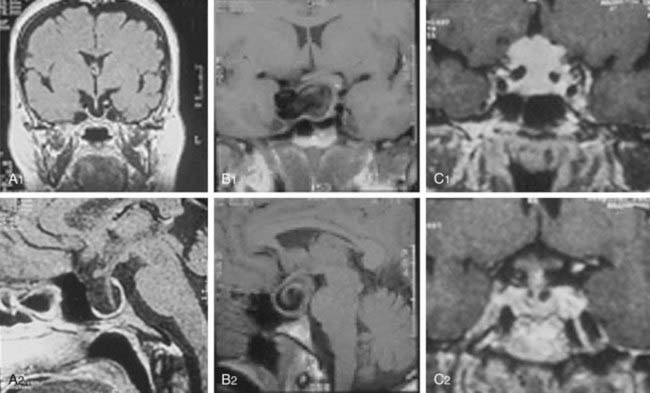
FIGURE 18-4. Magnetic resonance imaging of lesions (1, coronal views; 2, sagittal views) associated with hyperprolactinemia: “empty sella” (A1, A2), intrasellar aneurysm (B1, B2), and meningioma (C1, C2).
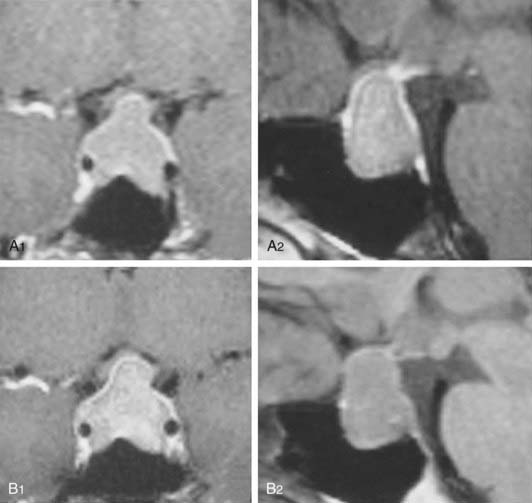
FIGURE 18-5. Magnetic resonance imaging of a 41-year-old woman with a nonfunctioning pituitary adenoma and serum prolactin level of 51 ng/mL before (A1, coronal view; A2, sagittal view) and during the 12th month of cabergoline “treatment” (B1, coronal view; B2, sagittal view). No tumor shrinkage was observed, despite serum prolactin decrease to 1.2 ng/mL.
(From Bronstein MD: Problems in the differential diagnosis of the hyperprolactinemic patient. Clinical Endocrinology Update 2003, syllabus pp 241–247; with permission of the Endocrine Society.)
Primary hypothyroidism can be associated with hyperprolactinemia, presumably due to high thyrotropin-releasing hormone levels that stimulate PRL release and presumably reduce prolactin metabolic clearance. Thyrotroph hyperplasia may occur, leading to pituitary enlargement mimicking a pituitary adenoma (see Fig. 18-3).35 Cushing’s disease and adrenal insufficiency can be associated with hyperprolactinemia, which also can be present in Nelson’s syndrome.49,50 Polycystic ovary syndrome also may be associated with hyperprolactinemia.51 Menstrual disturbances are prevalent in patients with polycystic ovary syndrome and in patients with prolactinomas, and sometimes the distinction between the two conditions may be difficult. The presence of mild hyperprolactinemia, negative pituitary imaging, high luteinizing hormone/follicle stimulating–hormone ratio, and clinical features suggestive of polycystic ovary syndrome can help in the differential diagnosis.
Uremia can be associated with hyperprolactinemia, mainly in patients with end-stage renal disease.52 The mechanism probably is related to reduced PRL clearance and to a presumably reduced dopaminergic tonus. Serum PRL elevation is mild but can be considerably increased in uremic patients taking drugs with a dopamine receptor–blocking effect. Hyperprolactinemia is present in up to 20% of patients with liver cirrhosis, probably due to an unbalanced estrogen-to-androgen ratio and to an altered dopaminergic tonus.53 Nipple manipulation and chest-wall lesions, such as herpes zoster and surgical scars, may increase serum PRL via stimulation of neuron pathways going through the spinal cord.54 In contrast to paraneoplastic adrenocorticotropic hormone (ACTH) secretion, ectopic production of PRL has rarely been described.55 Finally, when all the above-mentioned causes are ruled out, hyperprolactinemia is considered idiopathic or functional. It is likely, however, that most patients with this condition harbor small microprolactinomas that went undetected with less-sensitive imaging tools used in the past, such as hypocycloidal polytomography and computed tomography (CT) and even with MRI. If there is no radiologic evidence of a prolactinoma at initial diagnosis of hyperprolactinemia, however, an identifiable adenoma is unlikely to develop in the long-term follow-up.56 Nevertheless, a recent study by De Bellis et al.57 points out the presence of anti-pituitary antibodies in 25.7% of patients with idiopathic hyperprolactinemia versus 0% in those with microprolactinoma, suggesting an autoimmune etiology for a subset of cases of idiopathic hyperprolactinemia.
Diagnosis of Prolactinomas
CLINICAL FEATURES
Hyperprolactinemia and its impact on the gonadotropic axis is the hallmark of microprolactinomas and macroprolactinomas and their clinical manifestations. Macroprolactinomas also may cause neurologic and visual disturbances and impairment of other pituitary functions owing to the tumor-mass effect (Table 18-2).58 Loss of visual fields and impairment of visual acuity are the main ophthalmologic manifestations. Headache is the most common neurologic presentation; it is attributed to dural stretch or cavernous sinus invasion and less frequently as part of the clinical symptoms of pituitary apoplexy.59 There are a few reports of special types of headache, such as the SUNCT (short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing) syndrome secondary to prolactinomas, but the role of hyperprolactinemia as causative agent is obscure.60 Other rare presentations of invasive macroprolactinomas are hydrocephalus,61 neuropsychiatric manifestations, and otoneurologic manifestations.62,63
Table 18-2. Clinical Manifestations of Prolactinomas
| RELATED TO HYPERPROLACTINEMIA |
| RELATED TO TUMOR MASS EFFECT |
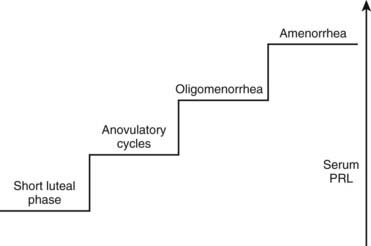
Women generally present with a correlation between the degree of serum PRL elevation and gonadal impairment, ranging from a short luteal phase to amenorrhea (Fig. 18-6). Most PRL-secreting pituitary adenomas are associated with amenorrhea, because hyperprolactinemia due to prolactinomas usually shows serum PRL levels greater than 100 ng/mL. Because hyperprolactinemia is present in about 20% of women with amenorrhea,64 most patients with such menstrual disturbance and high serum PRL levels may harbor a prolactinoma. Because microprolactinomas can be associated with serum PRL levels between 40 and 100 ng/mL, and because PRL biological activity does not always correspond to routine laboratory assay levels,65 some women with prolactinomas may exhibit milder forms of gonadotropic impairment such as anovulatory cycles and oligomenorrhea.66 In addition to menstrual disturbances, hyperprolactinemic women complain of loss of libido, vaginal dryness, dyspareunia, and psychological distress.67 Similar to other disorders associated with hypogonadism, osteoporosis is a frequent finding in women with hyperprolactinemia/prolactinomas, mainly in prolactinomas with long-standing hypogonadism. The relative risk of developing osteoporosis in premenopausal women with prolactinomas can be 4.5%.68 Although a direct effect of PRL on bone has been considered, there is evidence that hyperprolactinemia is not a risk factor in itself for the development of osteoporosis, with the associated hypoestrogenism being the major determinant.69
Galactorrhea is a frequent finding in women with hyperprolactinemia, but it can be absent, mainly in patients with macroprolactinomas associated with severe hypogonadism. Breast examination, if not adequate, also may fail to disclose mild galactorrhea. These facts may explain the discrepancy among series, which show a prevalence of galactorrhea ranging from 30% to 84%.64,70 Galactorrhea also may occur in normoprolactinemic women. A study including 235 patients with galactorrhea associated with diverse conditions showed that serum PRL was normal in 86% of women with idiopathic galactorrhea without amenorrhea.71 The mechanism is unknown, being attributed to different causes such as mammary hypersensitivity to PRL or “occult” transient hyperprolactinemia.
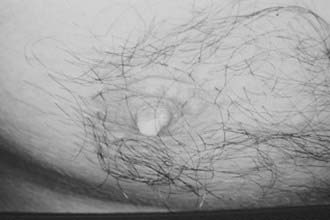
Hyperprolactinemia in men is predominantly associated with macroprolactinomas. The main clinical manifestations are hypogonadism, which is usually severe, along with loss of libido and reduced body hair growth, visual impairment, and headache.72 Only 22.5% of 80 patients with macroprolactinomas in our series sought medical assistance due to sexual complaints, whereas most patients requested appointments because of visual or neurologic disturbances. During the interview, 85% of the patients admitted, however, that loss of libido was of utmost importance.73 In patients with microprolactinomas (n = 12), sexual dysfunction was the main complaint in 67% of the cases, but that number increased to 92% after the interview.73 Testosterone replacement without serum PRL normalization seldom restores libido, an observation that points to a direct effect of PRL on sexual behavior, as previously suggested by animal models.74 Galactorrhea is far less frequent in men than in women with prolactinomas.75 It was disclosed in 15% and 25% of our patients with macroprolactinomas and microprolactinomas.73 When present in men harboring pituitary tumors, however, it strongly suggests the presence of a prolactinoma (Fig. 18-7).75 Osteoporosis also is present in hyperprolactinemic men.76
Although the prevalence of prolactinomas in both genders is higher in the 20s and 30s, prolactinomas can occur in elderly and in younger individuals. Data on 44 young patients (12 males and 32 females, aged 16.3 ± 1.9 years at diagnosis) with pituitary adenomas showed a predominance of macroadenomas (61%) over microadenomas (39%). Of those, prolactinomas were the most prevalent (68% of cases).77 Other series on prolactinomas diagnosed in childhood or adolescence showed that the prevalence of macroadenomas was also higher (15 versus 11 cases)78 or similar (24 versus 23 cases) compared with microadenomas.79 The predominance of larger tumors in children and adolescents points to molecular mechanisms influencing proliferation rather than the time course of the disease influencing the progression of prolactinoma size and invasiveness.
LABORATORY EVALUATION
Basal serum PRL evaluation usually confirms the clinical suspicion of a prolactinoma. Serum PRL usually ranges from 50 to 300 ng/mL in the presence of microprolactinomas and from 200 to 5000 ng/mL in the presence of macroprolactinomas (normal values range from 2 to 15 ng/mL). Values of 30 ng/mL have been associated with microprolactinomas, however, and values of 35,000 ng/mL have been found in patients harboring large and invasive macroprolactinomas. Stimulation tests with thyrotropin-releasing hormone and metoclopramide or suppression tests using levodopa, previously popular mainly for the differential diagnosis of microprolactinomas and so-called idiopathic hyperprolactinemia, give nonspecific results and have been largely abandoned.80
Hyperprolactinemia secondary to hypothalamus-pituitary disconnection rarely exceeds 150 ng/mL, and lesions that mimic macroprolactinomas, especially nonfunctioning pituitary adenomas, generally exhibit serum PRL less than this value (pseudoprolactinomas).46 A laboratory artifact may lead to an erroneous differential diagnosis between macroprolactinomas and pseudoprolactinomas, however. When serum PRL is evaluated by two-site immunometric assays, large amounts of antigen—PRL in this case—saturate capture and signal antibodies, impairing their binding and causing serum PRL to be underestimated (the so-called high-dose hook effect). Patients bearing macroprolactinomas with extremely high serum PRL levels (generally >10,000 ng/mL, depending on the assay measuring range) may present with falsely lower levels, within the 30 to 150 ng/mL range, causing the patient to be misdiagnosed as harboring a nonfunctioning pituitary adenoma. To avoid unnecessary surgeries (treatment of choice for nonfunctioning tumors), PRL assays with serum dilution or using two-step incubation are recommended in patients with macroadenomas who may harbor a prolactinoma.81
If such assays are not readily available, clinical clues pointing to prolactinomas are patient age younger than 50 years, presence of galactorrhea in male patients, and tumor shrinkage under dopamine agonist drugs, as shown by fast visual improvement in cases with chiasmal compression or rapid tumor reduction evidenced by MRI.75
Another laboratory pitfall concerns the presence of high serum PRL levels in subjects with few or no symptoms related to PRL excess. Human PRL in circulation manifests as marked size heterogeneity, with three forms (23 kD, 50 kD, and 150 to 170 kD) that are indistinguishable by routine assays.82 The 23-kD form (little PRL) is the most common form, but serum PRL can be elevated secondary to the presence of 150- to 170-kD aggregates with low biological activity (big-big PRL), leading to macroprolactinemia, a term coined by Jackson and colleagues83 in the 1980s. Less frequently, the 50-kD form (big PRL) can be the prevalent circulating form.84,85 The presence of molecular aggregates with low biological activity, such as big-big PRL, should be suspected when high serum PRL levels are detected in patients without or with scarce signs and symptoms related to hyperprolactinemia.4,86 Precipitation with polyethylene glycol is an excellent screening method.87 The predominant molecular form recovered (i.e., assayed after the precipitation) is the highly biologically active little PRL. The gel filtration chromatography confirms the presence of big-big PRL (Fig. 18-8), but being a costly and time-consuming method, it is performed for practical clinical purposes only when polyethylene glycol precipitation results are inconclusive. Macroprolactinemia is a common finding, occurring in 8% to 42% of all cases of hyperprolactinemia.4 The pathogenesis of macroprolactinemia is still unknown. It could be in part a complex of monomeric PRL with immunoglobulin G89; anti-PRL autoantibodies were identified in patients with idiopathic hyperprolactinemia.89
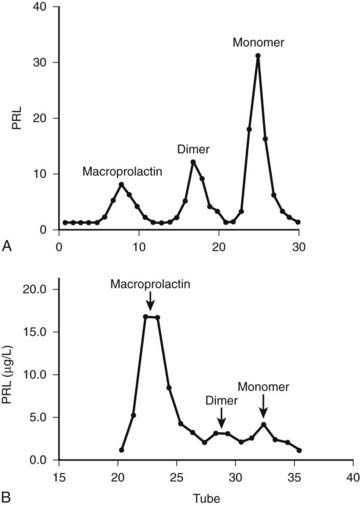
FIGURE 18-8. Gel filtration chromatography of a patient with symptomatic prolactinoma (A) and of an asymptomatic woman with macroprolactinemia (B). PRL, Prolactin.
Big-big PRL biological activity is still controversial in the literature. Studies in vitro with rat Nb2 cell bioassays show either the presence or the absence of biological activity.4 To explain the dissociation of presence of activity in vitro but not in vivo, we can speculate that because of its large molecular weight, macroprolactin does not cross the capillary barrier, and it is unable to reach target cells. Additionally, the PRL receptor forms of rat cells are different from those of humans, so a bioassay using cells harboring human PRL receptors which addresses the biological activity of macroprolactin should be of interest. As a matter of fact, a study by Glezer et al.90 showed that sera of individuals with macroprolactinemia presented lower biological activity in a bioassay using a mouse cell transfected with the long form of human PRL receptor as compared to the rat Nb2 bioassay (Fig. 18-9). Moreover, in a recent publication, Hattori et al.89 claim that the level of bioactivity of macroprolactin in the Nb2 bioassay is present due to the dissociation of monomeric PRL from the autoantibodies, as a result of the longer incubation and more dilute assay conditions. Despite these controversies in the literature concerning the biological activity of PRL aggregates, most patients with macroprolactinemia do not manifest clinical features related to hyperprolactinemia and do not need any treatment. To avoid unnecessary medical or surgical procedures, macroprolactin screening is mandatory when clinical features and serum PRL assay results are conflicting.
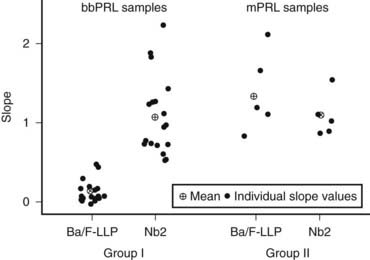
FIGURE 18-9. Individual slopes of bioactivity/immunoactivity (BA/IA) of macroprolactinemic individuals (group I) and hyperprolactinemic patients without macroprolactinemia (group II). The mean slope obtained in Ba/F-LLP assay for group I was significantly lower, compared to that obtained in the Nb2 assay for the same group. On the contrary, no significant differences between the mean slopes of both assays were found in group II.
(From Glezer et al: Human macroprolactin displays low biological activity via its homologous receptor in a new sensitive bioassay. J Clin Endocrinol Metab 91:1048–1055, 2006.)
Assessment of other pituitary functions is mandatory, mainly in the presence of macroprolactinomas. Insulin-like growth factor 1 must be followed either to verify GH deficiency or to disclose the rare cases of prolactinomas that progress with PRL/GH co-secretion.91 Gonadotropin assessment may explain cases with persistent amenorrhea or sexual impairment despite PRL normalization. Although less frequently affected, thyrotropic and adrenocorticotropic axes also must be evaluated. Thyrotropin assessment also may disclose primary hypothyroidism as the cause of hyperprolactinemia.35 Because restoration of pituitary function may occur after adenoma removal by surgery or tumor shrinkage attained by medical therapy,92,93 these assessments have to be repeated after such procedures to avoid unnecessary hormone replacement.
IMAGING
MRI is currently the gold-standard imaging method for diagnosis and treatment follow-up of micro- and macroprolactinomas, although CT scan still have a place for sellar region assessment. MRI is superior to CT for tumor boundary delineation, especially regarding its associations with the optic chiasm and cavernous sinus (Fig. 18-10). Additionally, tumor consistency, presence of hemorrhage, and presence of cystic lesions are better shown by MRI, especially comparing T1-weighted and T2-weighted images. CT has the advantage of showing bone erosion and calcifications and may help surgical planning.94 Although MRI is currently the most sensitive imaging method for microprolactinomas, tumors measuring less than 2 mm may remain undetected by this technique. This fact is well known for the usually tiny ACTH-secreting adenomas leading to Cushing’s disease, with about 50% of them not identified by MRI.95 It is probable that many of the so-called idiopathic hyperprolactinemic patients harbor a small microprolactinoma. Procedures such as dynamic imaging with gadolinium enhancement (Fig. 18-11) and spoiled gradient recalled acquisition technique (SPGR)96 may contribute to improve MRI sensitivity for such tiny adenomas.
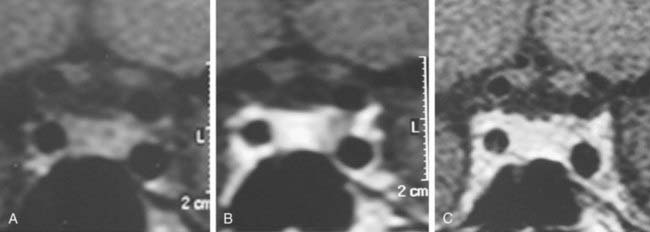
FIGURE 18-10. Magnetic resonance imaging (coronal views) of a microprolactinoma disclosed only during dynamic gadolinium-enhanced sequence. A, Before contrast injection. B, Lack of contrast enhancement on the left side of the pituitary gland suggestive of microadenoma. C, Whole gland contrasted.
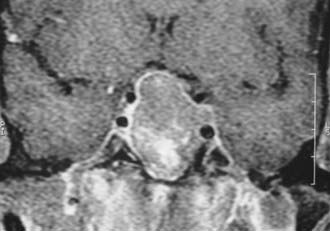
FIGURE 18-11. Magnetic resonance imaging (gadolinium-enhanced coronal view) showing optic chiasm compression and left cavernous sinus invasion by a macroadenoma.
MRI can disclose the so-called pituitary incidentaloma, a term coined by Reincke in 1990 to describe incidentally discovered pituitary masses by imaging performed to evaluate conditions not linked to pituitary disease, including head trauma and sinusitis. Controversies exist in the literature concerning the definition of pituitary incidentalomas.97 Molitch98 included patients who presented with symptomatic pituitary disease and who were diagnosed only when a sellar mass was incidentally disclosed. In our opinion, the term incidentaloma should be reserved for cases with no endocrine or mass effects of a pituitary adenoma.4 Incidental imaging findings of pituitary adenomas, mainly microadenomas, are present in up to 27% of autopsy findings in the general population.11 Other imaging pitfalls include normal anatomic variations such as asymmetric sphenoid septum with bulging of the sellar floor or displacement of the pituitary stalk,99 artifacts such as clips and prostheses, and global pituitary enlargement—either physiologic, as shown in puberty or even in young adults100 and pregnancy,101 or pathologic, as seen in primary hypothyroidism 35 and mental depression.102
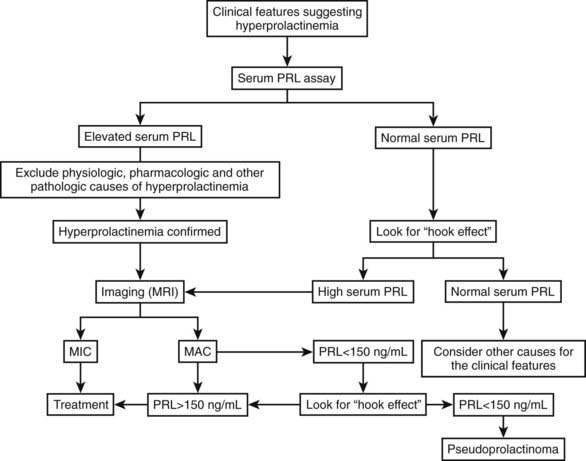
Regarding the functional evaluation of prolactinomas by imaging, in-vivo imaging by single-photon emission computed tomography using radiolabeled high-affinity benzamine derivatives to D2 receptors, such as iodine 123–methoxybenzamide or iodine 123–epidepride, could serve as a response predictor to dopamine agonist treatment.103,104 Positron emission tomography using 18-fluorodeoxyglucose, carbon 11–methionine, or dopamine D2 receptor ligands also has been used for in-vivo assessment of the metabolic rate and D2 receptor density of macroprolactinomas and other pituitary tumors.105 These methods are expensive and time consuming, however, and are better reserved for the study of tumors without a hormone marker, such as clinically nonfunctioning pituitary adenomas. Regarding prolactinomas, such techniques currently should be reserved for investigational purposes or for special cases, such as searching for metastases in the rare malignant prolactinomas.106 Fig. 18-12 is a flow diagram for the diagnostic evaluation of hyperprolactinemia.
Treatment
The therapy of hyperprolactinemia/prolactinomas aims at reverting the symptoms dependent on hormone hypersecretion and the neurologic and visual manifestations due to tumor-mass effect. The ideal treatment must spare or even improve other pituitary dysfunctions, if present. Good tolerance and low recurrence rates also are therapeutic goals.
Treatment of secondary hyperprolactinemia is intended to treat or remove the cause of the disorder. Levothyroxine usually corrects hyperprolactinemia associated with primary hypothyroidism. The surgical removal of a nonfunctioning pituitary adenoma with mass effect and withdrawal of drugs such as sulpiride and haloperidol, when possible, bring serum PRL down to normal levels.
As far as prolactinoma treatment is concerned, the 1970s brought almost concomitantly two powerful therapeutic advances: the improvement of pituitary microsurgery2 and the development of ergot derivatives with potent dopaminergic agonistic activity.107 Additionally, radiation therapy, including more recent stereotactic techniques, has a place, albeit restricted, in prolactinoma treatment (Table 18-3). Therapeutic strategy must consider several aspects, such as the patient’s clinical presentation, the differences between microadenomas and macroadenomas concerning their natural history, the desire for pregnancy, and the patient’s treatment preference, if applicable.
Table 18-3. Treatment of Prolactinomas
| MEDICAL |
| SURGICAL |
| RADIOTHERAPY |
SURGICAL THERAPY
Pituitary surgery by the transsphenoidal approach was used at the beginning of the 20th century and was reintroduced in the early 1960s and greatly improved when the surgical microscope was introduced.2,108 More recently, endonasal endoscopic surgery has become available. Compared to the classic transsphenoidal approach, endoscopic pituitary surgery seems to reduce the time of hospitalization, but improvement of surgical results remains to be demonstrated.109,110 These developments made the selective removal of the pituitary adenoma possible, sparing the normal gland, along with low complication and mortality rates, mainly for surgeons with more than 500 operations.111 Besides serum PRL normalization, this surgical modality aims at reducing or eliminating the mass effect of expanding macroadenomas, often leading to the resolution of neurologic and visual manifestations. The transcranial surgical approach is reserved solely for tumors with a predominance of extrasellar location expanding out of the midline.112
The surgical success in normalizing serum PRL levels depends on the experience and ability of the surgeon and on the tumor size and invasiveness. Preoperative serum PRL levels, usually associated with tumor dimension and location, were found to be paramount in predicting surgical remission, being the only predictive factor in multivariate analysis in some reports.113,114 Consequently, the best results were achieved in microprolactinomas with a preoperative serum PRL less than or equal to 100 ng/mL. A study on the initial outcome of 219 women with prolactinomas operated on by transsphenoidal microsurgery showed a remission rate of 92% of cases with preoperative PRL less than or equal to 100 ng/mL and 91% with intrasellar microadenomas, but only 59% in women with microadenomas with cavernous sinus extension, leading to an overall remission rate of 82% for microadenomas. Of the women with macroprolactinomas, 88% with intrasellar adenomas, 86% with moderate suprasellar extension, and 80% with focal sphenoid sinus invasion achieved remission. Surgical remission in patients with diffuse sphenoid sinus invasion, cavernous sinus invasion, and major suprasellar extension was poor, however, ranging from 0% to 44%.113 In another large series of 120 patients with prolactinomas (93 women and 27 men) who underwent pituitary surgery by the transsphenoidal route, PRL normalization occurred in 78% of patients with microadenomas, in 87.5% of patients with intrasellar macroadenomas, and in 27% of patients with extrasellar macroadenomas.114
A compilation of 50 published series showed that postsurgical serum PRL normalization was achieved in 73.7% of 2137 microprolactinomas and 33.9% of 2226 macroprolactinomas.115 Comparison among the series is difficult, however, because many authors do not mention preoperative serum PRL levels, the tumor size, and degree of invasiveness. The grade of clinical improvement in cured patients is high, mainly in smaller tumors. Menses and fertility restoration is high, and pregnancy rates ranged from 75% to 90% in different series. Some patients can achieve menstrual regulation and even become pregnant without full PRL normalization.114,116 Sexual improvement in men is less likely to occur, probably owing to irreversible gonadotropic damage caused by the higher frequency of extrasellar and invasive prolactinomas compared with women. Nonetheless, pituitary function generally is preserved in intrasellar adenomas and is restored by the removal of suprasellar adenomas causing hypothalamus-pituitary disconnection.114 In addition, neurologic and visual amelioration often is achieved after pituitary surgery.
Many patients bearing prolactinomas have undergone treatment with dopamine agonist drugs before surgery. The effect of previous medical therapy on surgical outcome is still a matter of debate. Some reports point to poorer results compared with nontreated patients117,118; others do not show significant differences.113,114 There is also evidence, however, of improvement of surgical results in the medically pretreated group.119,120 Because the issue of dopamine agonist–induced fibrosis remains unresolved, it is unclear whether the negative outcome was caused directly by the drug or whether it was biased by a tendency to treat medically large and invasive tumors, which present with the poorest surgical results.
An important caveat of surgical treatment is prolactinoma recurrence. This issue was raised in the early 1980s when an article from the Montreal group reported recurrence rates in surgically “cured” patients to be 50% for microprolactinomas and 80% for macroprolactinomas.121 Subsequently, several reports on this issue were published but with less impressive figures. A literature compilation reported recurrence rates of 18.2% of 809 microprolactinomas and 22.8% of 465 macroprolactinomas.115 In our series, the recurrence of hyperprolactinemia in surgically cured patients was 27% for microprolactinomas and 17% for macroprolactinomas.116 Median time to recurrence varied among the different surgical series, from 1 to 7 years. Concerning recurrence predictors, many studies pointed to higher postoperative serum PRL levels in patients who recurred compared with patients without recurrence,113,121,122 whereas others did not find serum PRL levels to be a predictor of late outcome.123 Another marker of recurrence is the absence of PRL response to dynamic tests, especially to thyrotropin-releasing hormone.114,123 These findings raise the question whether, in prolactinomas, serum PRL increase after surgical normalization represents true recurrence or important but incomplete tumor removal.
Although the occurrence of relapses contributes to the decrease of long-term surgical cure rate of prolactinomas, many patients with recurrent hyperprolactinemia remain asymptomatic and have no evidence of tumor regrowth.113,124 Relapse can be transient. From a cohort of 44 patients with surgically cured microprolactinomas, 8 (18.2%) experienced recurrence and were followed up. Only two of the eight patients experienced permanent relapse.125 Additional therapy is reserved only for patients with symptomatic recurrence.
Surgical treatment for prolactinomas is indicated for cases with persistent intolerance or hormonal or tumor resistance to dopamine agonists (see later); pituitary apoplexy126; tumor growth during medical therapy127; cerebrospinal fluid (CSF) leakage due to dopamine agonist–induced tumor shrinkage of invasive macroprolactinomas128,129; and (rarely) visual loss on medical therapy, secondary to optic chiasm herniation resulting from tumor retraction.130 Additionally, surgery is an excellent alternative for patients harboring microprolactinomas, especially patients with serum PRL less than or equal to 100 ng/mL, with poor compliance to medical therapy.113,131
MEDICAL THERAPY
The knowledge that dopamine is a powerful inhibitor of PRL secretion has yielded insights regarding use of dopamine agonists in hyperprolactinemia treatment.
Bromocriptine
At the end of the 1960s, an ergot derivative, 2-bromo-α-ergocryptine (bromocriptine) was developed, and shortly thereafter its use in clinical trials was initiated.107,132 Bromocriptine binds and stimulates the seven-membrane–spanning dopamine D2 receptors in normal and in adenomatous lactotrope cells, inducing activation of the Gi receptor (negatively coupled to adenylate cyclase) and, consequently, leading to postreceptor events that ultimately cause the inhibition of PRL synthesis and secretion.133 Although many other dopamine agonist drugs were developed thereafter, bromocriptine is still the one that presents the largest and longest worldwide experience (Table 18-4).
Table 18-4. Main Dopamine Agonist Drugs
| Drug | Usual Dose |
|---|---|
| Bromocriptine | 2.5-10 mg/day |
| Pergolide | 0.025-0.5 mg/day |
| Quinagolide* | 0.075-0.6 mg/day |
| Cabergoline | 0.5-2 mg/wk |
* Not approved in the United States.
Pharmacokinetic studies with bromocriptine show that after a single oral dose of 2.5 mg, serum levels peak, and maximal suppressive action occurs between 1 and 3 hours. The drug has a relatively short mean elimination half-life (about 6.2 hours) and generally is administered twice a day.134 Owing to a considerable interindividual variability in the PRL-lowering effects of a given dose of bromocriptine, however, some patients need to take the drug three times a day and others just once a day, mainly when higher doses are required or normoprolactinemia already has been achieved.135
Bromocriptine treatment results in serum PRL normalization and clinical improvement in most patients with hyperprolactinemia/prolactinomas. A compilation of 13 series from the literature, encompassing 286 women treated with bromocriptine, showed PRL normalization and return of menses from 64% to 100% and 57% to 100% of cases.134 Our data showed evidence of normoprolactinemia in 55% of patients with microprolactinoma treated primarily or postsurgically with bromocriptine (mean dose 3.8 mg/day), with menses return in 98% of the patients. Of women without PRL normalization, 81% also recovered menses, making unnecessary the increase of bromocriptine dose, optimizing drug tolerance and reducing costs.116 Although less frequent, microprolactinoma in men also can be treated successfully with dopamine agonists. Of 12 patients predominantly treated with bromocriptine, 83% achieved normal PRL level and clinical improvement.135 Eleven men with microprolactinoma have been treated by us, with serum PRL and serum testosterone normalization in 73% and 86% of them.73
Because macroprolactinomas often present with mass effect, they were first surgically treated and, if not cured, then treated with bromocriptine. The observation by Corenblum and colleagues136 in the mid-1970s that bromocriptine use reduces tumor size in addition to its PRL-lowering effect, consequently relieving neurologic and visual complaints, also allowed primary treatment for macroprolactinomas. In a prospective multicenter trial, normal PRL levels were achieved in 18 of 27 patients (67%) followed up for at least 12 months while receiving variable doses of bromocriptine.137 All patients exhibited some degree of tumor shrinkage: 9, less than 25%; 5, between 25% and 50%; and 13, greater than 50% tumor size reduction. Another study with macroprolactinomas with extrasellar extension showed serum PRL normalization in all of the 10 men and 17 of 19 women, with mean bromocriptine doses of 13 mg/day and 8 mg/day. Tumor size was reduced by more than 50% in 18 of 29 patients (62%) with a secondary empty sella in 5 cases and by less than 50% in 11 patients. Visual field improved in most of the patients who initially presented with such abnormalities.138 Patients with giant prolactinomas (diameter >4 cm) also achieved disease control with primary bromocriptine therapy.139 In our series, serum PRL was normalized in 60% of women with macroprolactinomas (mean bromocriptine dose 7 mg/day); 72% of these women had menses recovery. Menses also were restored in 44% of patients who remained hyperprolactinemic.116 The lower rate of menses normalization compared with women harboring microprolactinomas is probably due to the higher prevalence of gonadotropic impairment in the macroprolactinoma group. Regarding macroprolactinoma in men, serum PRL and testosterone levels normalized in 67% and 85% of 66 patients bearing macroprolactinomas, showing that in men also, the gonadotropic axis may normalize even when PRL is not fully normalized.73 Other series show different figures, with PRL returning to normal levels in 83% of patients but with testosterone normalization in only 62% of them, probably reflecting different degrees of gonadotropic impairment. We obtained 80% of tumor reduction in primarily treated macroprolactinoma patients.
The mechanism of prolactinoma shrinkage by dopamine agonists is not yet fully understood. Some reports in the early 1980s suggested that tumor reduction was due to lactotrope cell size reduction.140 Bromocriptine decreases mRNA and PRL synthesis within days and cell multiplication (antiproliferative and proapoptotic mechanisms have been suggested) and tumor growth.141 These events are evidenced quickly microscopically by a decrease in the number of PRL secretory granules, involution of the rough endoplasmic reticulum and Golgi apparatus, and decrease of cytoplasmic volume. With longer periods of treatment (e.g., 6 months), there is evidence of cell vacuolization and fragmentation with collagen deposition.142 These early and late morphologic changes of the tumor lactotropes may explain the rapid regrowth of macroprolactinomas when bromocriptine is discontinued after a short period of therapy,143 whereas often no tumor expansion is observed when the drug is withdrawn after being used for a longer period.144 Serum PRL levels may be suppressed in some patients without tumor shrinkage, although the converse does not occur.
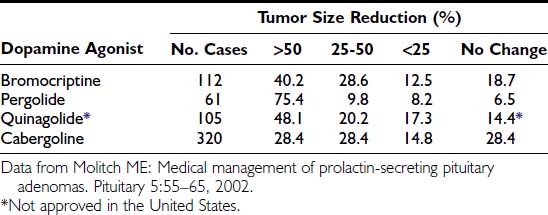
The degree of macroprolactinoma reduction by bromocriptine and its time course have been carefully analyzed.145 Data on 271 patients included from prospective series on primary therapy of true macroprolactinomas showed that 79% of patients presented with more than 25% shrinkage and that 89% of them had shrunk to some degree. Pretreatment serum PRL concentration and gender difference did not predict the degree of tumor reduction. Of 102 prolactinomas large enough to produce chiasmal compression, 85% showed tumor shrinkage of more than 25%. Another compilation, including eight series from the literature totaling 112 patients with macroprolactinomas,146 showed that 40.2% had a greater than 50% reduction in tumor size; in 28.6%, the reduction was 25% to 50%; in 12.5%, the reduction was less than 25%; and 18.7% had no evidence of tumor size reduction (Table 18-5). The time course of macroprolactinoma shrinkage is highly variable. Some patients experience a dramatic improvement of visual acuity 12 hours after bromocriptine has been introduced, with tumor shrinkage being documented within 1 week. In others, significant tumor reduction is observed only 1 year after medical therapy has been started. In the U.S. multicenter study cited previously,137 19 patients had tumor shrinkage in 6 weeks, but in 8 others, the reduction was not observed until the 6-month imaging reassessment was performed. General data from most series show that rapid shrinkage occurs during the first 6 months in most cases, with slower reduction thereafter, and additional decreases in tumor size observed after 1 year for several years in some cases. Visual improvement generally parallels and often precedes tumor shrinkage unless the optic tract has been chronically and severely damaged. Fig. 18-13 illustrates an unusual case of long-term macroprolactinoma shrinkage, showing that minor tumor reduction, even when not depicted by imaging, is sufficient for visual field improvement. In such situations, the clinical amelioration signified that the primary medical therapy was effective even without clear tumor shrinkage, and pituitary surgery could be postponed or, as it was in this case, needless.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree