FIGURE 68-1. Hip fracture incidence as a function of BMD measured at the hip, spine, and forearm in postmenopausal women.
(Data from Cummings SR, Black DM, Nevitt MC, et al: Bone density at various sites for prediction of hip fractures, Lancet 341:72–75, 1993.)
It is estimated that with this 2.5 SD threshold, 30% of white women older than 50 years have osteoporosis,6 a fraction that is similar to the lifetime risk of fracture at the hip, spine, and forearm for a 50-year-old woman.7 By this definition, about 0.6% of young adult women have osteoporosis, and 16% have low bone mass. The prevalence of osteoporosis increases with age because of the decline in bone mass. Not all women who have osteoporosis by the WHO criteria will sustain fractures, and conversely, many women who do not have osteoporosis by this definition may deserve treatment because of other risk factors that will increase their risk of fracture. Thus, this operational WHO definition of osteoporosis provided the concept of osteoporosis as a risk factor for fracture, which is important for assessing the number of affected individuals, but patient diagnosis must include other risk factors for fracture. This is the role of the individual fracture risk assessment of fracture.
Recently, a WHO Scientific Group issued a technical report including hierarchical levels of evidence for the ability of risk factors to identify individuals at risk for fracture.8 Those risk factors were validated by meta-analyses of population-based cohorts from Asia, Australia, Europe, and North America. Those risk factors were BMD at the femoral neck, low body mass index, a prior fragility fracture, glucocorticoid exposure, a parental history of hip fracture, smoking, excessive intake of alcohol, and rheumatoid arthritis. The combination of those risk factors allowed for building fracture probability models, providing individual probabilities of fracture that will be the basis for therapeutic decision making, thus shifting the former therapeutic threshold paradigm, which was often based only on the BMD T score.
Epidemiology
MAGNITUDE OF THE PROBLEM
In women, osteoporosis is rare before menopause. With aging, more and more women are affected by osteoporosis; by the age of 80 years, 27% have low bone mass and 70% have osteoporosis at the hip, spine, or forearm.7 Sixty percent of osteoporotic women will experience one or more fragility fractures. In the United States, it is estimated that 16.8 million (54%) of postmenopausal white women have low bone mass, and 9.4 million (30%) have osteoporosis.7 These rates of prevalence are lower in nonwhite women and in men. NHANES III (Third National Health and Nutritional Examination Survey) data indicate that 10,103,000 Americans (8,021,000 women and 2,082,000 men) have osteoporosis and that 18,557,000 (15,434,000 women and 3,123,000 men) have low bone mass and thus an increased risk of osteoporosis in comparison to those who do not have low bone mass.8 The prevalence of vertebral fractures can vary according to the definition of fracture used. Thus 10% to 25% of women older than 50 years are estimated to have vertebral fractures.9,10 In 1990, 1.66 million hip fractures were estimated worldwide in those older than 35 years.11
Osteoporosis results in high rates of morbidity. In 1986 in the United States, osteoporosis was responsible for an estimated 321,909 hospitalizations in white women 45 years and older—167,421 for hip fractures, 35,106 for fragility vertebral fractures, 120,636 for wrist fractures, and 20,369 for humerus fractures.12 Osteoporosis-related fractures also significantly contribute to the economic burden of health care. In 1995, direct costs for osteoporosis fractures were estimated to be $13.8 billion,13 whereas it was 5 to 6 billion annually 10 years earlier.12 Being elderly, patients with hip fractures often have other medical conditions, thus increasing their risk of complications such as pressure sores or infections. Hip fractures often result in admission to nursing homes.14 The number of hip fractures and their associated costs are expected to sharply increase and could triple by the year 2040. Indeed, the number of elderly people is quickly growing, and fracture incidence rates increase with age.15 This phenomenon is observed in developed countries, but the projected rapid expansion of the elderly populations of Latin America and Asia could lead to an increase in hip fractures from 1.66 million worldwide in 1990 to 6.26 million in 2050, with only 25% occurring in North America and Europe.11 Osteoporosis is also associated with mortality. For each standard deviation decrease in bone mineral density, the mortality risk is increased 1.5-fold.
The lifetime risk of fractures can be estimated, given the probability of fracture and the odds of reaching a given age. Most data have been generated in white populations of Western countries. The lifetime risk of sustaining an osteoporotic fracture has been estimated in the United States to be close to 40% for women and 13% for men. For vertebral fractures, the estimated risk is 15.6% for women and 5% for men. The risk of hip fracture is 17.5% for women and 6% for men and for wrist fracture, 16% for women and 2.5% for men.7
SITE-SPECIFIC FRACTURES
Hip Fracture
Hip fracture is the most serious complication of osteoporosis. It results in high morbidity and sometimes mortality. The most striking characteristic of hip fractures is that they are associated with a 12% to 20% reduction in expected survival.16 Many of the deaths after hip fractures are related to other diseases.17 Few deaths are actually due to or hastened by the fracture, but the risk of dying of another disease is sometimes increased by the hip fracture. About 5% of women sustaining a hip fracture die during the following year as a consequence of fracture. In women living in nursing homes, 36% of those having a hip fracture will die within a year. Thirty percent to 50% of these patients are unable to regain the level of function they had before the hip fracture. The patient’s physical condition before the fracture may be the best predictor of postfracture functional outcome.
The incidence of hip fracture increases exponentially with age in both genders. In women younger than 35 years, the incidence is 2 per 100,000 person years, whereas it is 3032 per 100,000 person years in women older than 85 years.18 In men the rates are 4 and 1909 per 100,000 person years, respectively. Most hip fractures occur in elderly people: 52% after age 80 years and 90% after age 50 years.18 The decline in BMD and the increase in frequency of falls in elderly people are responsible for this high incidence of hip fracture. Only 1% of falls lead to a hip fracture, but 90% of hip fractures are related to a fall from standing height or less.19 Hip fractures tend to be the consequence of falls on hard surfaces, the fall not being stopped by protective reflexes of upper limbs.20 Fifty percent of the falls leading to hip fractures result from slipping or tripping, 20% from loss of consciousness, and 20% to 30% of loss of balance and other factors.
Vertebral Fracture
The epidemiology of vertebral fractures is less well characterized than that for hip fractures because symptoms after vertebral fracture are unidentified in up to 70% of cases, and standardized radiographic diagnostic criteria for vertebral fractures are lacking. Less than a third of patients with vertebral deformities seek medical assistance, and 2% to 8% need hospital admission.23 In women, 90% of vertebral deformities are a consequence of mild to moderate trauma, whereas this proportion is only 50% in men.24
The incidence of clinical vertebral fractures increases gradually with age in both genders. The age-adjusted prevalence is thought to be between 8% and 25% in women older than 50 years. The difference in prevalence figures is due to the definition used.24 Early definitions of vertebral fracture were based on the type of deformity and thus had poor precision. New techniques using vertebral morphometry give more reliable results.25 Vertebral deformities are mostly noted in women, with a ratio of 2 : 1, but this ratio becomes narrower in elderly people older than 80 years. It has been suggested in a large epidemiologic study involving 15,570 European men and women that the incidence of vertebral deformities might be equal in both genders. In the same cohort, vertebral fracture incidence in women over 50 was estimated at 10.7/1000 person years in women and 5.7/1000 per person years in men.26 Changes in the gross appearance of the vertebral body encompass a wide range of morphologic characteristics, from increased endplate concavity to complete destruction of the vertebral anatomy in vertebral crush fractures.27 It must be kept in mind that vertebral deformity is not synonymous with vertebral fracture; for example, Scheuermann’s disease is frequently discussed as a differential diagnosis.28
New vertebral fractures, even those not recognized clinically, are associated with substantial increases in back pain and functional limitation due to back pain, at least in elderly women.29 Ten percent to 20% of elderly women with worsening of their functional status because of back pain have had a vertebral fracture. Common complications of vertebral deformities are chronic back pain, back disability, height loss, limitations in activity, emotional difficulties stemming from physical appearance, and more medical consumption. Kyphosis from vertebral fractures adds to the overall back pain. For instance, after adjustment for age, a 15% increase in kyphosis increases the odds ratio of severe upper back pain 2.1-fold. However, no association has been found between the number of fractures and impairment in quality of life. The occurrence of vertebral fracture is predictive of new fracture, with RR = 5 to 11. Vertebral fractures are associated with increased mortality at 5 years, as for hip fracture. The increase in mortality is progressive over this period, as opposed to hip fracture. The risk of death appears to be much higher for clinically apparent vertebral fracture, and the presence of multiple vertebral fractures further increases this risk.30,31
Wrist Fracture
Wrist fractures, also called distal forearm fractures or Colles’ fractures, are very common. The incidence of wrist fracture is hard to calculate because only a minority of patients is hospitalized. A Norwegian study showed that it was the most common fracture responsible for admission to a local university hospital.32 Incidence varies among different ethnic groups. Wrist fracture risk is lower in black people than white people.33 Increasing incidence was observed between 1950 and 1982,34 but this trend seemed to have leveled off during the second part of the 1980s.
The pattern of incidence of wrist fracture is different from that of hip or vertebral fractures. The age-adjusted female-to-male ratio is 4 : 1, with a linear increase in incidence in women from ages 40 to 60 years, followed by a plateau.35 In men, the incidence is almost constant between the ages of 20 and 80 years, for unknown reasons. Wrist fractures are not associated with an increase in mortality and are usually thought to be free of long-term poor outcome.36 Other data, however, suggest that half of the patients do not report good functional status at 6 months because of complications such as algodystrophy, neuropathies, and posttraumatic osteoarthritis.37
Wrist fractures must be considered as an important predictor of subsequent vertebral and hip fractures. Indeed, it has been shown that men and women with distal forearm fractures have on average a twofold increase in the risk of sustaining a hip fracture.38 Previous wrist fractures are also significant predictors of overall osteoporotic fractures, with a doubled risk. In younger women, the predictive value of a previous wrist fracture for subsequent osteoporotic fractures seems even more important, with a relative risk of 2.67 (1.02 to 6.94) for women aged 40 to 49 years. This predictive value is of the same magnitude as that provided by a 1-SD decrease in BMD measured by bone densitometry.39 Thus, the occurrence of a wrist fracture in an early postmenopausal woman is suggestive of osteoporosis and should trigger appropriate investigation such as BMD measurement.
Other Fractures
Other fractures include proximal humerus, pelvis, proximal tibia, and rib fractures. An excess of these fractures is noted in postmenopausal women, their rates increase with aging, and they are often a consequence of minimal trauma. These fractures are related to low bone mass, similar to hip and vertebral fracture, and they should also motivate BMD measurement because any of them could be the first symptom of osteoporosis.
VARIABILITY IN FRACTURE INCIDENCE
The incidence of hip fracture is markedly lower in black and Asian people and varies among different countries and populations. Rates are higher in Scandinavia than in Western Europe and Oceania.21 A north-south gradient in age-standardized risk is found in Europe and the United States,22 with a higher rate in the north. The age-adjusted increase in incidence that has been observed in several countries over the past 50 years appears to have leveled off in some of these countries. The incidence increases with socioeconomic difficulties, decreased winter sunlight, and water fluoridation. Fractures are more frequent in winter months. This seasonal trend could be due to altered neuromuscular coordination and vitamin D deficiency in winter months, because most fractures occur indoors and thus cannot be explained only by slippery winter conditions.22 About 80% of hip fractures occur in women, because the age-adjusted incidence in men is two times lower than in women, and more elderly women are alive than are elderly men.
COSTS
In the United States, direct medical expenditures for osteoporotic fractures were estimated at $20 billion in 2000,40 two-thirds of which are accounted for by hip fracture alone. An important determinant of rising cost is increased age. The estimated cost for care in the United States during the first year after a hip fracture is $21,000 per person and is much higher in women older than 80 years. If the cost of the subsequent nursing home is attributed to the hip fracture, the cost of care for those who stay in nursing homes after 1 year is $93,378 per person. The average cost for vertebral fracture during the first year is $1200 per patient, and it is $800 for Colles’ fracture. Costs are expected to double in 2050, due to the increasing number of fractures resulting from the aging population.
OSTEOPOROSIS: A NEGLECTED DISEASE
It has been shown in recent years that even after marketing of several effective therapies for osteoporosis, most patients who have sustained an osteoporotic fracture (forearm fracture, vertebral fracture, and even hip fracture) do not receive adequate secondary prevention for future fractures, whereas the risk of repeated fractures is markedly increased, including that of second hip fracture.41
Pathogenesis
Osteoporosis is a multifactorial disease. Most but not all risk factors influence the level of bone mass. Some may have an impact on bone structure (the so-called quality of bone), and some increase the risk of fragility fracture through extraskeletal mechanisms. One should distinguish risk factors for falls from risk factors for bone fragility.
CLINICAL RISK FACTORS
Clinical risk factors can shed light on the pathophysiology of fragility fractures. Bone mineral density is a key risk factor for fracture,42 but numerous studies have evaluated other risk factors for fractures.16,43–50 The use of such factors (Table 68-1) to identify high-risk subjects is frequently advocated. Different types of fractures have different risk-factor profiles.44
Table 68-1. Risk Factors for Hip Fracture With and Without Adjustment for Calcaneal Bone Density and History of Prior Fracture
| Factor | Relative Risk (95% Confidence Interval) | |
|---|---|---|
| Base Model | Adjustment for Fracture and BMD | |
| Age (per 5 yr) | 1.5 (1.3-1.7) | 1.4 (1.2-1.6) |
| History of maternal hip fracture | 2.0 (1.4-2.9) | 1.8 (1.2-2.7) |
| Increase in weight since age 25 yr | 0.6 (0.5-0.7) | 0.8 (0.6-0.9) |
| Height at age 25 yr | 1.2 (1.1-1.4) | 1.3 (1.1-1.5) |
| Self-rated health | 1.7 (1.3-2.2) | 1.6 (1.2-2.1) |
| Previous hyperthyroidism | 1.8 (1.2-2.6) | 1.7 (1.2-2.5) |
| Current use of long-acting benzodiazepines | 1.6 (1.1-2.4) | 1.6 (1.1-2.4) |
| Current use of anticonvulsant drugs | 2.8 (1.2-6.3) | 2.0 (0.8-4.9) |
| Current caffeine intake | 1.3 (1.0-1.5) | 2.0 (0.8-4.9) |
| Walking for exercise | 0.7 (0.5-0.9) | 1.2 (1.0-1.5) |
| On feet <4 hr/day | 1.7 (1.2-2.4) | 0.7 (0.5-1.0) |
| Inability to rise from chair | 2.1 (1.3-3.2) | 1.7 (1.2-2.4) |
| Lowest quartile for distant depth perception | 1.5 (1.1-2.0) | 1.4 (2.0-1.9) |
| Low-frequency contrast sensibility | 1.2 (1.0-1.5) | 1.2 (1.0-1.5) |
| Resting pulse rate >80 beats/min | 1.8 (1.3-2.5) | 1.7 (1.2-2.4) |
| Any fracture since age 50 yr | 1.5 (1.1-2.0) | |
| Calcaneal bone density (per 1-SD decrease) | 1.6 (1.3-1.9) | |
BMD, Bone mineral density.
Data from Study of Osteoporotic Fractures.
Estrogen Deficiency
Estrogen deficiency has been associated with osteoporosis since first suggested by Fuller Albright in the 1940s. It is the main cause of bone loss in women after menopause. Premature menopause—natural or surgically induced—extends the period during which a woman is exposed to a hypogonadal state, thus increasing the total duration of bone loss occurring after menopause.51 Similarly, late menarche and primary or secondary amenorrhea also increase the risk for osteoporosis.52 Luteal deficiency in premenopausal women has been suggested to be associated with bone loss,53 but the evidence is still controversial. Estrogen deficiency is also associated with bone loss during the 3 to 4 years preceding the actual menopause—the perimenopause—with a rate similar to that observed in the early postmenopausal years.54 It is likely that women with the lowest concentrations of residual estrogen have faster bone loss and fracture more often, although this might be confounded by the effect of body weight or other sex steroids.55–57 Also, women receiving aromatase inhibitors as adjuvant therapy for breast cancer lose bone more rapidly and sustain an increased rate of fragility fracture.58
Other Factors
Many factors increase the risk of fracture. For instance, the Study of Osteoporotic Fractures49 identified 16 independent risk factors for hip fracture in addition to low BMD (see Table 68-1). Numerous studies have described risk factors such as female gender, white or Asian race, age, previous fractures, thinness, cigarette smoking, family history of hip fracture, use of sedative hypnotics, and impairment in visual and neuromuscular function. Lack of physical activity may also be an important cause of bone loss,59,60 and it has been hypothesized that some of the age-related bone loss and the burden of skeletal fragility result from a decline in physical activity, in particular, in Western societies. Black people have less osteoporosis and fewer fractures than white and Asian people do. Excessive alcohol consumption increases the risk of fractures, whereas moderate intake might exert a protective effect. Undernutrition in the elderly may contribute to bone loss, the risk of falling, or the response to injury.61 It is widely believed that inadequate calcium intake throughout life is a significant risk factor.62
In a cohort of 7575 French women aged 75 years or older, the risk of subsequent hip fracture was significantly increased (even after adjustment for femoral hip BMD) in women with a slow gait speed, in women who had difficulty performing a tandem (heel-to-toe) walk (i.e., neuromuscular impairment), and in women with visual acuity less than 2/10.63 The incidence of hip fracture among women classified as being at high risk because of both a high fall risk status and a low BMD was found to be 29 per 1000 women years. It was 11 per 1000 in women at risk because of either a high fall risk status or low BMD, and the risk was 5.4 per 1000 women years in those at low risk according to both criteria. Even with this combined approach, about a third of women with a hip fracture had not been identified as being at high risk, thus indicating that other factors are important in the pathogenesis of hip fracture. Although these clinical risk factors can be easily assessed with a rapid questionnaire and physical examination, they are probably of limited value in younger women. A personal history of hip fracture is still predictive, but its prevalence is much lower in women younger than 65 years. Fall-related factors have not been tested but are likely to be poor discriminants in younger and healthier women.
Candidate risk factors have been examined by a WHO working group in individual data meta-analyses of 12 prospective population-based cohorts. Data were collected from 60,000 men and women participating in the Rotterdam study in the Netherlands; the European Vertebral Osteoporosis Study and the European Prospective Osteoporosis Study; the Canadian Multicenter Osteoporosis Study; the Dubbo Osteoporosis Epidemiology Study in Australia; the EPIDOS and OFELY cohorts in France; and cohorts from Rochester, Minnesota; Sheffield, England; Kuopio, Finland; Hiroshima, Japan; and Gothenburg, Sweden. Several risk factors have been selected on the basis of the possibility to modify them and ease of use in clinical practice: BMD at the femoral neck, low body mass index, a prior fragility fracture, glucocorticoid exposure, a parental history of hip fracture, smoking, excessive intake of alcohol, and rheumatoid arthritis.64–68 Those factors are modeled to provide individual probabilities of fracture that can be easily calculated using the computer-driven WHO fracture risk assessment tool called FRAX (available at Internet websites such as http://www.shef.ac.uk/FRAX/).69
PEAK BONE MASS
Peak bone mass is the amount of bone acquired at the end of skeletal growth, and it is followed by bone loss throughout the rest of life. Bone mass at a given age is a function of the peak bone mass achieved and the amount of bone lost as a consequence of menopause and aging. Peak bone mass, which is reached in early adult life, primarily depends on genetic factors. It is also influenced by dietary calcium intake during adolescence and by physical activity.
Blacks achieve higher peak bone mass than whites do, who have greater peak bone mass than Asians, particularly Japanese. Twin studies have shown a strong correspondence of peak bone mass in monozygotic twins, who have closer concordance in BMD than dizygotic twins.70 Which genes are the most important is still unknown. It has been reported by Morrison et al. that polymorphism of a noncoding sequence of the vitamin D receptor gene was associated with peak bone mass variance in monozygotic twins, and that these polymorphisms accounted for most of the genetic effect.71 This association has not been confirmed by most subsequent studies72–74 but has initiated searches for other candidate genes, including the estrogen receptor, interleukin 6 (IL-6), transforming growth factor β (TGF-β), and type I collagen. For example, the collagen Iα1 Sp1 polymorphism is moderately associated with BMD and fracture, as shown in a meta-analysis of 13 studies involving 3642 patients.75 More recently, some variants of LRP5 have been found associated with BMD and fracture.76–78 Even if many advances have been made in understanding the role of genetic factors in the last 10 years, a great deal of additional research is required to identify the genes relevant to the pathogenesis of osteoporotic fracture. Indeed, most studies so far have been underpowered. In the future, it is likely that a combination of large-scale studies and technologic improvements such as genomewide associations will help identify candidate genes in the regulation of bone strength.77–79
Dietary calcium intake may be important for attaining optimal peak bone mass. Three placebo-controlled trials in children or adolescents, including one performed in twins, have shown a modest but significant increase in BMD in those receiving calcium supplementation.80–82 Children with very low calcium intake are more likely to benefit from an increase in calcium, preferably from a dietary source.
Other factors may be important, such as those influencing the progression of puberty. Exercise during growth may contribute to the level of peak bone mass. It has been shown that intensive exercise before puberty may enhance bone acquisition that might persist in adulthood,83 but the role of exercise in the normal physiologic range is unknown. In girls, areal BMD gains stop around ages 16 to 20 and in boys around ages 20 to 22. Bone width, however, will continue to increase throughout life, even after linear growth cessation, to maintain bending strength despite areal BMD decline.84 Diet, physical activity, and toxic substances exposure, including maternal cigarette smoking during pregnancy (programming), may also influence the level of peak bone mass.85,86
BONE LOSS WITH AGING
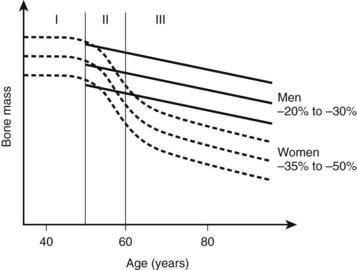
Changes in bone mass as a function of age are presented in Fig. 68-2. Throughout life, women lose 35% to 50% of their bone mass, depending on the skeletal site. Although osteoporosis is a systemic disease affecting the whole skeleton (with the exception of the bones of the skull and face), the pattern of bone loss differs slightly and depends on the type of bone. It has been generally believed that bone loss starts around menopause. In fact, a recent study showed that trabecular bone loss—assessed at the distal forearm with high-resolution peripheral quantitative computed tomography (HR-pQCT)—starts in young adults of both sexes, accounting for a third of the total trabecular bone loss over the entire life.87 Menopause induces accelerated bone loss within 5 to 8 years, followed by a linear rate of bone loss that may accelerate after the age of 75 years.88 Because of a much slower rate of bone remodeling, cortical bone loss may start later, but the total amount of cortical bone mass that is lost in women in their 80s is probably similar to the amount of cancellous bone lost. The proportion of overall bone loss related to menopause and to the estrogen-independent aging process is debated, but clearly bone loss in the elderly is still under the influence of estrogen deficiency and can be prevented by hormone replacement therapy (HRT).89
Two major mechanisms underlie bone loss in women. First, an age-related decrease in osteoblast activity occurs and leads to an imbalance between the amount of bone resorbed and the amount of bone formed within a remodeling unit (BMU). Second, menopause induces a marked increase in the number of remodeling units activated within the cancellous and cortical bone envelopes per unit of time, which will amplify the small deficits observed at each BMU. This overall increase in bone turnover peaks within the first 2 to 3 years after menopause but persists throughout life, even in women in their 80s, as shown by the sustained increase in bone markers of resorption and formation in elderly postmenopausal women. The mechanism by which estrogen deficiency results in increased bone resorption and bone loss is not yet completely understood, but evidence indicates that estrogens act indirectly through cytokines and growth factors such as IL-1α, IL-1 receptor antagonist, tumor necrosis factor α (TNF-α), IL-7, and TGF-β. Estrogen also stimulates production of osteoprotegerin—a decoy receptor that is a potent inhibitor of osteoclast activity—in osteoblastic cells and is also an inhibitor of receptor activator of nuclear factor κB (RANKL) and RANKL-stimulated osteoclastogenesis, thus explaining a large part of the antiresorptive action of estrogen in bone.90–92 Estrogen results in an increase in IL-7 in target organs such as bone, thymus, and spleen, at least in part through decreases in TGF-β and increased IGF-1 production, leading to a first wave of T-cell activation.93 Those activated T cells release IFN-γ, increasing antigen presentation by dendritic cells and macrophages as a result of up-regulation of MHCII expression through the transcription factor CIIA.94 Estrogen deficiency also amplifies osteoclastogenesis and T cell activation by down-regulating antioxidant pathways, resulting in an upswing in ROS, which in turn stimulates antigen presentation and TNF production by mature osteoclasts. As a result, T-cell activation is again amplified and promotes release of the osteoclastogenic factors RANKL and TNF. In addition, TNF stimulates osteoblastic RANKL and M-CSF production, in part by up-regulating IL-1, thus leading to osteoclastic formation.95,96 TNF and IL-7 also directly blunt bone formation through their effects on osteoblasts.
Bone turnover in osteoporosis, however, varies widely with patients with high, normal, and low bone turnover.97 In osteoporosis, trabecular plates are perforated and transform into rods, leading to thinning of trabeculae and loss of connectivity, with increased risk of fracture as a result. Cortices also thin because of reduced periosteal apposition failing to compensate for bone loss at the endosteal surfaces. Modifications in collagen matrix (e.g., variations in the proportions of various crosslinks and isomerization of C-telopeptide) and the degree of mineralization may also influence bone strength.
Serum intact parathyroid hormone (PTH) levels increase with advanced age in both genders. This hyperparathyroidism is secondary to the calcium and/or vitamin D deficiency commonly found in the elderly, especially in those institutionalized, and may contribute to bone loss in both women and men. A classification of osteoporotic fractures based on clinical features and underlying mechanisms has been used. Type I osteoporosis includes mainly wrist and vertebral fractures, occurring mostly in women younger than 70 years, and is predominantly due to loss of cancellous bone because of estrogen deficiency. Type II osteoporosis includes mainly hip fractures that occur in both elderly men and women as a result of cancellous and cortical bone loss driven primarily by secondary hyperparathyroidism. It has been proposed that this model should be unitary, with bone loss caused by estrogen deficiency in both phases and in both genders.43 In this model, the accelerated phase of bone loss after menopause involves a disproportionate loss of cancellous bone, mainly due to estrogen deficiency. Compared to normal postmenopausal women, osteoporotic women seem to have impaired responsiveness to postmenopausal low levels of estrogen. The subsequent phase of slow bone loss involves proportionate losses of cancellous and cortical bone and is associated with secondary hyperparathyroidism. In aging men, low testosterone and estrogen levels contribute to bone loss. In the elderly, decreased bone formation also contributes to bone loss, probably due to stem cells which tend to differentiate toward the adipocyte lineage rather than the osteoblastic lineage.
BONE QUALITY
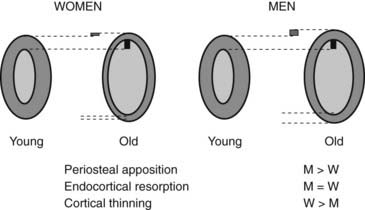
Bone strength is determined by its material composition and structure.98 Bone must be stiff to resist deformation, thereby making loading possible, and it must also be flexible to absorb energy by deforming. Bones shorten and widen when compressed, and lengthen and narrow in tension. When bone is brittle (i.e., too stiff and unable to deform a little), the energy imposed during loading leads to structural failure, initially by the development of microcracks and then by complete fracture. When bone is too flexible and deforms beyond its peak strain, it breaks. Long bones are mainly made of cortical bone, favoring rigidity over flexibility, whereas mainly trabecular vertebrae can absorb more energy by deforming more before breaking. Differences in bone dimensions explain the better tolerance to load in men compared with women and in some races compared with others.99 Men and women generally have similar vertebral trabecular volumetric density and similar vertebral heights. The larger vertebral cross-sectional area in men contributes to sex-based differences in bone strength. Black people tend to have wider but shorter vertebral bodies and higher trabecular volumetric density than do white people, owing to thicker trabeculae. The geometry of the hip (hip axis length) influences the risk of hip fractures. Femoral neck length is a predictor of future fracture, and individuals with particularly long femoral necks are more likely to have hip fractures. This feature has been noted in white women from the United States100 and France.101 The increase in height over the second part of the 20th century could partly explain the increase in the incidence of hip fracture during this period.102 Bone modeling (construction) produces a change in size and shape of bone when new bone is deposited without previous bone resorption. During bone remodeling (reconstruction), resorption by osteoclasts precedes bone formation by osteoblasts. Bone modeling and remodeling modify the external size and contours of bone and its internal architecture by the deposition or removal of bone from the surface of bone. They result in cortical and trabecular thickening during growth and thinning during aging. Bone loss occurring with menopause and aging is associated with disturbances in bone microarchitecture.103 Osteoclastic resorption leads to focal perforations in cancellous bone plates, which results in loss of connection of the horizontal plates, along with detachment of vertical bars throughout the bone marrow cavity. Thus the probability of crush fracture is increased in bones rich in cancellous bone, such as vertebrae. The thickness of cancellous bone plates is about 100 to 150 µm, and osteoclasts dig resorption defects of 50 to 100 µm during normal remodeling. Perforations in cancellous plates can be a consequence of increased osteoclast activity and lead to impairment in bone mechanical properties and therefore to an increased risk of fracture. With continued remodeling, trabeculae perforate and some disappear completely. Active endocortical and intracortical remodeling “trabecularizes” cortical bone (i.e., creates cortical bone with more surface area).104 Older, more densely mineralized interstitial bone distant from surface remodeling has a reduced number of osteocytes and accumulates microdamage.105 Both cortical thinning and cortical porosity reduce the resistance of bone to the propagation of cracks, leading to complete fracture in case of a fall. The loss of bone strength due to cortical thinning and porosity is partially offset by deposition of new bone on the external surface (periosteal apposition), so cortical thickness is better maintained than would occur without periosteal apposition (Fig. 68-3).106,107 However, details of changes in periosteal apposition with aging—along with the effects of site, sex, and race—are difficult to evaluate prospectively, given the small magnitude of periosteal apposition throughout adult life. The notion that periosteal apposition is greater in men than in women remains controversial.108 More recent studies suggest that sex-based differences may occur at some but not all sites.99,109 Sex-based differences may also vary across races.
ROLE OF FALLS
Skeletal determinants of bone strength do not reflect all the factors related to fracture risk. For any given bone density, the risk of fracture is greater in the elderly. The increased frequency of falling, the type of fall that occurs among the elderly, and the loss of protective soft tissue may all explain the larger contribution of age and the less important role of bone mass in the elderly. Among postmenopausal women in the United States, the frequency of at least one annual fall rises from about one in five at 60 to 64 years of age to one in three at 80 to 84 years of age.110 Propensity for falling has been assessed in several studies48 with parameters such as gait speed, inability to rise from a chair without using one’s arms, and of course, visual impairment. These parameters are associated with a risk of falling. The increase in falling is nevertheless not sufficient to account for the increasing incidence of fractures, because only 5% to 6% of falls result in a fracture (1% of hip fractures and 4% to 5% for other fractures). Fracture risk is also related to the seriousness of the trauma on the femur and the direction of the fall. Indeed, the risk of hip fracture is 13 times higher when the impact is delivered directly over the hip.110 A great amount of force can be dissipated by the thickness of soft tissue over the femur, and patients with low fat mass may be at higher risk of hip fracture.
Clinical Features
POSTMENOPAUSAL OSTEOPOROSIS
The most common clinical form of osteoporosis is postmenopausal. Typically, women sustain a wrist fracture about 10 years after menopause, a vertebral fracture 15 to 20 years after menopause, and eventually, after 75 years of age, a hip fracture.
Wrist fractures or distal forearm fractures are mainly of two types: a Colles’ fracture is a consequence of dorsal angulation, and the less frequent type, a Smith fracture, results from volar angulation. These fractures usually have a favorable outcome, but some patients suffer from algodystrophy, osteoarthritis, or neuropathies.36
Two types of hip fracture are cervical and trochanteric. The femoral trochanter is composed of more cancellous bone than the femoral neck is. It seems that the predictive value of BMD and ultrasonic parameters may be higher for trochanteric fracture than for cervical fracture.111 Hip fractures are associated with more morbidity and mortality, and the prefracture functional state is restored in less than half of patients (see earlier in Epidemiology).
Vertebral fracture requires separate consideration. Vertebral fracture results in back pain, which often appears after some strain on the back, such as lifting a suitcase or working in the garden. The pain is commonly severe and often confines the patient to bed. This pain localizes to the back and rarely radiates to the legs; cord compression is exceptional, and in this latter case, one must consider other diagnoses such as metastases or myeloma. Pain from the fracture usually eases over a period of 6 to 8 weeks and disappears. Nevertheless, it has been estimated that about two-thirds of vertebral fractures are not diagnosed at the time they occur because they are considered by patients or physicians as nonspecific back pain. Loss of height is another main feature of vertebral fracture, but often patients do not report it spontaneously, so it needs to be sought by asking about the individual’s height in early adulthood and measuring the current height. Therefore, it is worthwhile to record the patient’s height at each clinical visit. Height loss of 3 cm or more should alert the physician to the possibility of a new vertebral fracture. Detection of asymptomatic vertebral fractures is clinically relevant because they are associated with a threefold to fivefold increased risk of new vertebral fractures, independent of the level of BMD. In addition, the incidence of new vertebral fracture in the year following a vertebral fracture has been estimated to be 20%.112 Kyphosis is generally a consequence of vertebral crush fractures in the thoracic spine and sometimes results in decreased lung capacity. Vertebral fractures in the lumbar spine result in decreased abdominal volume, which causes protrusion of the abdomen and impingement of the costal margin on the iliac crest. This iliocostal contact provokes pain and a grating sensation.
DISEASES AND TREATMENTS CONTRIBUTING TO OSTEOPOROSIS
A variety of disorders are associated with an increased risk of osteoporosis (Table 68-2). In most of these cases, osteoporosis appears to be multifactorial and cannot be attributed to only a specific disease (“secondary osteoporosis”). We will discuss only the most common disorders associated with osteoporosis.
Table 68-2. Diseases and Treatments Contributing to Osteoporosis
Glucocorticoid-Induced Osteoporosis
Consistent evidence indicates that glucocorticoids impair bone formation113 by directly inhibiting osteoblastic activity. Osteoblastic synthesis of type I collagen, osteocalcin, and alkaline phosphatase is decreased; the production of insulin-like growth factor 1 (IGF-1) and IGF-2 is also inhibited by glucocorticoids. Measurement of serum osteocalcin provides a good index of this osteoblast inhibition by glucocorticoids. The effect of corticosteroids on bone resorption is less clear. High doses have been associated (in some but not all studies) with an increased rate of bone resorption resulting from decreased intestinal calcium absorption, increased urine calcium excretion, decreased osteoprotegerin levels, and hypogonadism. Glucocorticoids contribute to adrenal and gonadal deficiency because of inhibition of pituitary hormone secretion, with a dose-dependent reduction in free testosterone in men. Consequently, exposure to supraphysiologic doses of glucocorticoids leads to substantial and rapid bone loss in most individuals, especially in the first year of therapy and with doses above 7.5 mg of prednisone. Thus fractures are very common, especially vertebral fractures, which occur in 30% to 50% of corticosteroid-treated patients; the risk of vertebral and hip fractures is generally more than doubled.114 Patients undergoing organ transplantation may have a higher fracture risk than other steroid-treated patients, perhaps because of the preexisting condition and the osteopenic effect of other immunosuppressive drugs. One of the main problems with corticosteroid treatment is the minimal effective dose to avoid side effects. Some evidence suggests that corticosteroid-induced inhibition of bone formation occurs even with low doses of inhaled corticosteroids, with a marked decrease in serum osteocalcin.115 With oral corticosteroids, the increase in fracture risk starts as low as 2.5 mg/day.116 Data from cross-sectional and prospective studies suggest that the bone mass of asthmatic patients treated with inhaled corticosteroids is lower than that of controls, in a dose-dependent fashion.117 The increased fracture risk rapidly offsets on cessation of therapy.118 BMD values are not good predictors of fracture risk in patients on corticosteroids, since the risks start to rise with T score around 0.0.119
Endocrine Diseases
Sex hormone deficiency in both genders results in bone loss. All diseases and drugs that reduce sex hormone levels are associated with bone loss, and the list includes athletic amenorrhea, anorexia nervosa, hemochromatosis, Turner’s syndrome, Klinefelter’s syndrome, numerous chemotherapeutic regimens, hypopituitarism, or treatment with luteinizing hormone–releasing hormone analogs for endometriosis. In type 1 diabetes mellitus, small and variable decreases in bone density have been reported. In type 2 diabetes, BMD seems to be increased, perhaps because of increased body weight and hyperinsulinemia, but fracture risk seems to rise, perhaps due to impairment in bone quality. Cushing’s syndrome leads to bone loss that is reversible after treatment of the disease. Thyroid hormones are major activators of bone remodeling.
Patients with hyperthyroidism are subject to high-turnover osteoporosis with or without mild hypercalcemia, and they have an increased risk of fracture.49 The decrease in BMD in thyrotoxic patients is reversible after treatment of the hyperthyroidism.120 The deleterious effects of supraphysiologic doses of thyroid hormone on bone may also occur (but to a lesser extent) in patients who receive suppressive doses of thyroxin for the treatment of thyroid carcinomas and nontoxic goiter. Conversely, hypothyroidism does not seem to significantly affect BMD. The potential role of calcitonin deficiency in bone loss after thyroidectomy is unclear.
Studies by DXA in mild primary hyperparathyroidism have shown that bone density is reduced in regions of cortical bone but is normal in areas of cancellous bone. This decrease in BMD is likely to increase the risk of fracture. Skeletal recovery after surgical treatment of parathyroid adenoma is very significant, with an increased BMD of about 6% at the femoral neck and 8% at the spine 1 year after surgery.121This improvement is sustained 10 years after surgery. These patients were the more severely affected patients whose surgery was dictated by the guidelines of the National Institutes of Health.
Gastrointestinal Diseases
All diseases associated with impairment in calcium and/or vitamin D absorption may induce bone loss and include disorders such as celiac disease, inflammatory bowel syndromes, jejuno-ileal bypasses, pancreatic insufficiency, gastrectomy, chronic liver diseases, or prolonged total parenteral nutrition. The decrease in bone density is sometimes due to osteomalacia rather than osteoporosis per se. The origin of gastrointestinal-induced osteoporosis can be multifactorial and could include, for example, the role of corticosteroid therapy in the case of inflammatory bowel diseases.
Bone Marrow Diseases
Multiple myeloma is generally characterized by osteolytic lesions but often induces generalized bone loss. Vertebral crush fractures are also very common. Histomorphometric studies of bone have shown a marked uncoupling between increased resorption and decreased formation that may be due to the secretion of IL-1, IL-6, Dickkopf 1 (DKK1), and TNF-β by plasma cells and other cells of the marrow environment. Corticosteroid therapy and immobilization can also contribute to this bone loss. Acute leukemia in children and adolescents, rather than lymphomas, is sometimes associated with generalized osteoporosis with or without osteolytic lesions. Systemic mastocytosis is a rare disease caused by the proliferation of mast cells infiltrating the skin, bone marrow, spleen, liver, and lymph nodes. Skeletal involvement may be focal or generalized, and about 70% of patients have radiographic abnormalities, including osteosclerotic lesions but also generalized osteopenia and vertebral fractures. In the absence of typical skin lesions, bone biopsy is often the only way to diagnose mastocytosis.
Other Conditions
Rheumatologic conditions such as rheumatoid arthritis and ankylosing spondylitis are associated with osteoporosis and an increased rate of fracture. Pregnancy is very uncommonly associated with bone loss in the last trimester. At intakes higher than 40 g of ethanol per day, alcoholism increases the risk of osteoporotic fractures, particularly in men. In addition to the direct effect of alcohol on osteoblasts, the role of hypogonadism and liver disease may be of importance. Caffeine consumption has also been associated with reduced bone mass and hip fracture risk in some studies.49,59 Medroxyprogesterone acetate is a progestational agent that suppresses gonadotropin, thus causing anovulation and hypoestrogenemia. Its prolonged use is associated with decreased spinal bone density in about 10%. Other drugs, such as some anticonvulsants, methotrexate, heparin, and cyclosporine, may increase the risk of osteoporosis. More recently, aromatase inhibitors58 and the thiazolidinediones used in the treatment of type 2 diabetes have been found to increase fracture risk.122
OSTEOPOROSIS IN YOUNG ADULTS
A few young adults have osteoporotic fractures corresponding to either mild osteogenesis imperfecta or idiopathic osteoporosis. Osteogenesis imperfecta is an inherited syndrome characterized by fragile bones and recurrent fractures that can lead to skeletal deformities.123 Inheritance and phenotypic expression are very heterogeneous. Clinical features of osteogenesis imperfecta also include short stature, blue sclerae, dentinogenesis imperfecta, hearing loss, scoliosis, and joint laxity. Osteogenesis imperfecta generally results from mutations of type I collagen.
Idiopathic juvenile osteoporosis is a very rare condition123 of children and adolescents before puberty. This disease does not seem to be of familial origin. Vertebral fractures usually occur over a 2- to 4-year period. In severe cases, patients may have deformities of the extremities and kyphoscoliosis.
Idiopathic adult osteoporosis is more often recognized because bone densitometry is more widely available. Although the condition may resemble mild osteogenesis imperfecta, these patients do not have dentinogenesis imperfecta, blue sclerae, or hearing loss. They do, however, have joint laxity and mild scoliosis, and a familial history is sometimes found.123
OSTEOPOROSIS IN MEN
Fractures are more prevalent in men than in women from childhood to middle life, probably because of a higher incidence of trauma. After 40 years of age, fractures are less common in men than in women, but the incidence of fracture as a result of mild trauma increases with aging. The incidence of hip fracture in men rises exponentially with age, as in women. The sex ratio (female to male) is about 2 : 1 in northern Europe, but it may vary in other areas and reflects the lower life expectancy of men. Mortality related to hip fracture is significantly higher in men than in women. Although vertebral deformities are common in men, many of them are unrelated to osteoporosis. Vertebral fractures in men are associated with height loss, kyphosis, and increased disability, as in women. The incidence of osteoporotic vertebral fractures seems to be half that in women.23 Vertebral fracture in men is associated with lower BMD than in controls.124 The incidence of limb fracture begins to rise at a later age in men than in women.
As in women, osteoporosis in men can result from an inadequate peak bone mass and/or accelerated bone loss. As discussed later, gonadal status may be critical for the achievement of peak bone mass in the male. Age-related bone loss is less pronounced in men than in women, around 15% to 20% from 30 to 80 years of age. The mechanisms underlying bone loss with aging in men are unknown, but some evidence indicates decreased osteoblastic activity in males with idiopathic osteoporosis.125 In elderly men, secondary hyperparathyroidism may contribute to bone loss.
The same risk factors for fragility fractures have been described in men and women and include smoking and excessive alcohol intake; these factors are often combined in a man with osteoporosis. About 50% of men with osteoporosis are considered to have secondary osteoporosis. The most common causes are chronic glucocorticoid therapy, hypogonadism, alcoholism, gastrectomy, and other gastrointestinal disorders. Male hypogonadism has a major influence on the occurrence of osteoporosis. Peak bone mass may be impaired by disorders of puberty, and men with abnormal or delayed puberty have reduced bone mass.126 Estrogen status is believed to be critical for the acquisition of peak bone mass in men since the observation that aromatase deficiency in a man, resulting in estrogen deficiency, led to the absence of epiphyseal closure and to osteopenia, abnormalities that can be successfully treated with estrogen.127 Androgens are also essential for the maintenance of bone mass in adult men insofar as hypogonadism in men is associated with low bone mass. Osteoporosis is encountered in many forms of hypogonadism, such as hyperprolactinemia, castration, anorexia, hemochromatosis, and Klinefelter’s syndrome. Prolonged abuse of alcohol has detrimental effects on the skeleton, with reduced bone mass resulting mainly from impaired osteoblast activity, nutritional deficiencies, and hypogonadism. Gastrointestinal disorders, particularly gastrectomy, are also associated with osteoporosis in men. Some but not all studies have linked nephrolithiasis or hypercalciuria with reduced bone mass, but their impact on the mechanisms of bone loss remains unclear.
The absolute risk of various osteoporotic fractures is similar in men and women with the same BMD level, but fewer men than women have a low BMD, and men are generally older if they reach such low levels.128 This difference in age and biomechanical factors such as bone size may also modulate the influence of BMD on fracture risk. There is currently no consensus on T scores that may be used as thresholds for therapeutic decision making in men. The −2.5 cutoff T score (if the reference values are obtained from a male population) is often used, given the similar relationship between BMD and fracture in men and women. The FRAX calculator69 can also be used in men to assess the individual probability of fracture, but the T score to enter in the software must be calculated using a reference curve obtained with women data (NHANES III database).
Pathology
HISTOMORPHOMETRY
Bone histomorphometry of the iliac crest allows an assessment of bone structure and turnover and is usually performed on transiliac bone biopsy samples. The specimen is processed without prior decalcification and analyzed with standardized histomorphometric methods. Histomorphometry is the only method to differentiate the cell and tissue level of remodeling. Nowadays, noninvasive techniques such as DXA and bone markers are sufficient for most clinical situations involving osteoporosis, and very few indications for bone biopsy still remain. Histomorphometry should be restricted to patients whose history, examination, radiographs, or biochemical profile suggests the possibility of atypical osteomalacia, mast cell bone disease, nonsecreting myeloma, sarcoidosis, or other rare conditions.
Diagnosis
Investigation of patients with osteoporosis is intended to fulfill the following purposes:
DIFFERENTIAL DIAGNOSIS AND CAUSES OF OSTEOPOROSIS
Evaluation of a patient suspected of having osteoporosis includes an adequate history and physical examination, and it must assess the potential causes of secondary osteoporosis and diseases mimicking osteoporosis. Biological abnormalities are commonly found in osteoporotic women, but few major medical problems are identified with screening,129 and routine laboratory testing may not be cost effective.130 However, a biochemical profile is necessary in patients with vertebral fractures, including assays of serum calcium, phosphate, creatinine, 25-hydroxycholecalciferol [25(OH)D], and alkaline phosphatase; serum protein electrophoresis; test for proteinuria; urine calcium; and in some cases, urine protein immunoelectrophoresis and blood cell count. Thyroid-stimulating hormone measurement and, in men, total and bioavailable testosterone and prolactin assays may be useful in some cases. In the elderly, 25(OH)D and PTH assays are appropriate when vitamin D deficiency is suspected. Assessment is guided by the clinical findings because some patients with apparent primary osteoporosis turn out to have mild hyperparathyroidism, osteomalacia, systemic mastocytosis, or late appearance of atypical osteogenesis imperfecta.
A complete examination of the spine is often necessary in patients with osteoporosis and includes height measurement and assessment of pain, paraspinal muscle contraction, thoracic kyphosis and lumbar lordosis, scoliosis, and the gap between the costal margin and the iliac crest, as well as the abdomen protrusion that can result from multiple vertebral fractures. Blue sclerae, joint hyperelasticity, and signs in favor of hyperthyroidism or hyperadrenocorticism should be looked for. A general examination is also important to rule out a neoplasm (e.g., lymph node and breast palpation) or other conditions.
RADIOLOGIC EVALUATION
The estimation of spine BMD on conventional radiographs is insensitive and inaccurate because the subjective assessment is dependent on radiographic exposure, patient size, and film-processing techniques. BMD has to decline by as much as a third before it can be detected on plain radiographs. The radiographic manifestation of osteopenia of the axial skeleton includes increased radiolucency of the vertebrae, sometimes vertical striation, framed appearance of the vertebrae (“empty box” or “picture framing”) and increased concavity of the vertebral endplates resulting from protrusion of the intervertebral disk into the weakened vertebral body (Fig. 68-4).131 Those signs of osteopenia, however, do not reflect the bone mineral status accurately, so they have been replaced by bone densitometry.
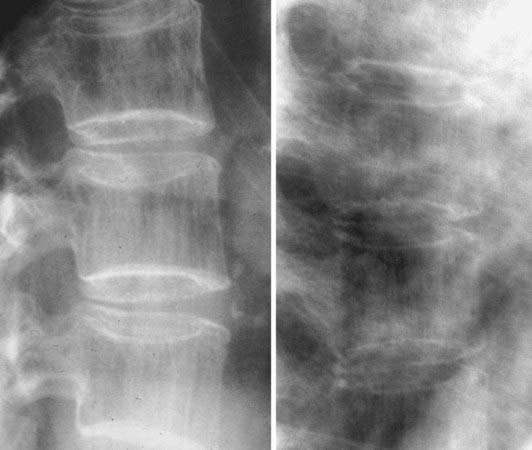
FIGURE 68-4. Reinforcement of the vertical primary trabeculae and loss of the horizontal trabeculae in postmenopausal osteoporosis led to vertical striations on a radiograph of the lumbar and thoracic spine, combined with an overall radiolucency and “empty box” appearance.
Radiographs are important tools to confirm fractures and their potential etiology. Plain radiographs with anteroposterior and lateral views are required for both the thoracic and lumbar spine. Vertebral fractures include wedge deformities, endplate (“biconcave”) deformities, and compression (or crush) fractures (Fig. 68-5). Some vertebral deformities unrelated to osteoporosis include Scheuermann’s disease, malignancies, and osteomalacia. The diagnosis of mild vertebral fracture can be difficult because of the overlap with the normal range of vertebral body shape. Algorithms to define vertebral fractures that were developed for epidemiologic studies and clinical trials have led to a consensus proposal for the radiologic diagnosis of vertebral fractures.132
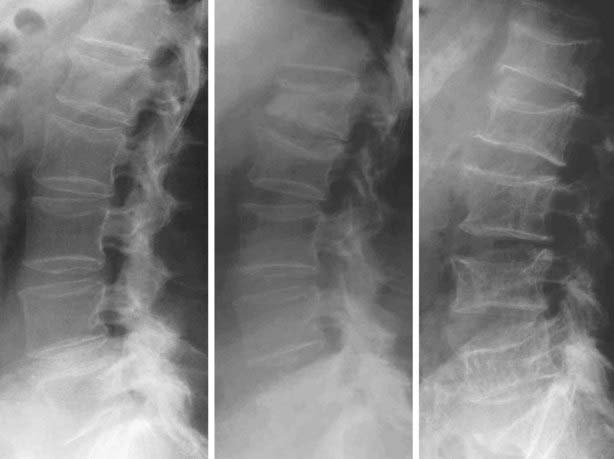
FIGURE 68-5. Progressive evolution of spinal osteoporosis in a postmenopausal woman with vertebral fracture cascade, at baseline, 10, and 20 years.
Vertebral fracture evaluation, however, may be challenging because of a considerable variability in fracture identification if clinicians or radiologists interpret radiographs without specific training. Several qualitative and quantitative methods have been developed to standardize visual vertebral fracture evaluation, but the most widely used currently is Genant’s semiquantitative assessment.133 Vertebral fractures are distinguished from other nonfracture deformities, and the severity of the vertebral fracture is assessed by visual determination of the magnitude of vertebral height reduction and morphologic change. The type of deformity (wedge, biconcave, compression) is not linked to the extent of vertebral height reduction—that is, the grading of the fracture. Vertebrae from T4 to L4 are graded on visual inspection as normal (grade 0), mildly deformed (grade 1, 20% to 25% in anterior, middle, and/or posterior height), moderately deformed (grade 2, 25% to 40% in anterior, middle, and/or posterior height), and severely deformed (grade 3, >40% in anterior, middle, and/or posterior height) (Fig. 68-6). Semiquantitative interpretation has excellent intraobserver and interobserver precision, provided careful training and standardization have been applied.133,134
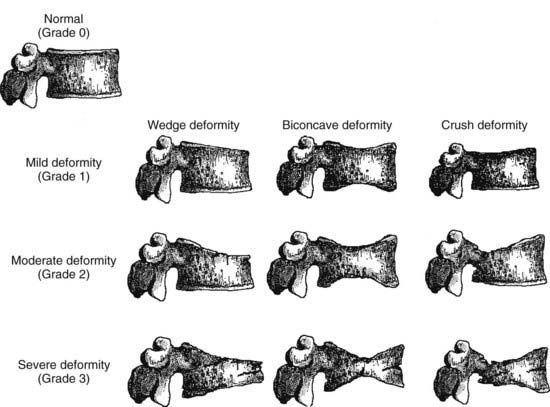
FIGURE 68-6. Schematic representations of wedge, biconcave, and crush vertebral deformities with grading normal to severe deformity. The figure illustrates the semiquantitative visual grading system of Genant.
(Drawn by C.Y. Wu.)
The axial skeleton is not the only site where changes due to osteoporosis can be observed. Widening of the medullary canal and thinning of the cortices is commonly described in older individuals as a result of longstanding endosteal bone resorption in the appendicular skeleton (Fig. 68-7). Conventional radiographs can also be used to assess the bone structure, with image procedures relying on texture or fractal analyses.135
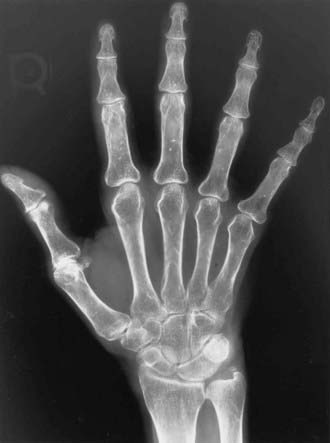
FIGURE 68-7. PA radiograph of the hand in an elderly osteoporotic woman showing severe diffuse cortical thinning, principally by endosteal resorption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree