Fig. 16.1
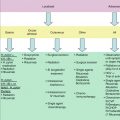
Fundoscopic examination of a patient with Waldenström’s macroglobulinemia demonstrating hyperviscosity-related changes including dilated retinal vessels, peripheral hemorrhages, and “venous sausaging” (Courtesy of Marvin Stone M.D.)
16.3.2 Immunophenotype
The lymphoid component of the tumor is positive for mature B-cell antigens, such as CD19, CD20, and CD79a, and expresses monotypic surface immunoglobulin light chain. These cells are most often negative for CD5 and CD10. The plasmacytic component of the tumor is positive for plasma cell antigens such as CD38 and CD138 and expresses monotypic cytoplasmic immunoglobulin light chain. In contrast to most cases of multiple myeloma, the neoplastic plasma cells of LPL are negative for CD56 (Leo et al. 1992; San Miguel et al. 2003).
16.3.3 Genetics
There are no chromosomal translocations associated with LPL, but a subset of cases show loss of chromosome 6q (Chang et al. 2007). Recently mutations in MYD88 have been discovered in the majority of LPLs – a finding that might prove useful for distinguishing LPL from marginal zone lymphoma (Treon et al. 2012).
16.3.4 Differential Diagnosis
Distinguishing LPL from marginal zone lymphoma (MZL) with plasmacytic differentiation can be difficult based on morphology and immunophenotype alone (Owen et al. 2003a). However, the systemic nature of the disease and the marked serum IgM paraprotein can usually rule out the latter diagnosis. Cases of LPL with a predominantly lymphocytic component often raise the diagnostic possibility of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). However, LPL lacks the characteristic proliferation centers and expression of CD5 typical for CLL/SLL. Finally, LPL with extensive plasma cell differentiation can raise the possibility of a plasma cell neoplasm. The demonstration of monotypic surface immunoglobulin expression on the B-cell population and the predilection of LPL for lymphoid tissues facilitate this distinction.
16.4 Clinical Features
It should be noted that most patients with WM will have limited and nonspecific symptoms at diagnosis, such as fatigue and malaise. Unlike most indolent lymphomas, splenomegaly and lymphadenopathy are prominent in only a minority of patients (≤15 %). Purpura is frequently associated with cryoglobulinemia and more rarely with AL amyloidosis, while hemorrhagic manifestations and neuropathies are multifactorial (see later). The morbidity associated with WM is caused by the concurrence of two main components: tissue infiltration by neoplastic cells and, more importantly, the physicochemical and immunological properties of the monoclonal IgM. As shown in Table 16.1, the monoclonal IgM can produce clinical manifestations through several different mechanisms related to its physicochemical properties, nonspecific interactions with other proteins, antibody activity, and tendency to deposit in tissues (Merlini et al. 1986; Farhangi and Merlini 1986; Marmont and Merlini 1991).
Table 16.1
Physicochemical and immunological properties of the monoclonal IgM protein in Waldenström’s macroglobulinemia
Properties of IgM monoclonal protein | Diagnostic condition | Clinical manifestations |
|---|---|---|
Pentameric structure | Hyperviscosity | Headaches, blurred vision, epistaxis, retinal hemorrhages, leg cramps, impaired mentation, intracranial hemorrhage |
Precipitation on cooling | Cryoglobulinemia (type I) | Raynaud’s phenomenon, acrocyanosis, ulcers, purpura, cold urticaria |
Autoantibody activity to myelin-associated glycoprotein (MAG), ganglioside M1 (GM1), sulfatide moieties on peripheral nerve sheaths | Peripheral neuropathies | Sensorimotor neuropathies, painful neuropathies, ataxic gait, bilateral foot drop |
Autoantibody activity to IgG | Cryoglobulinemia (type II) | Purpura, arthralgias, renal failure, sensorimotor neuropathies |
Autoantibody activity to red blood cell antigens | Cold agglutinins | Hemolytic anemia, Raynaud’s phenomenon, acrocyanosis, livedo reticularis |
Tissue deposition as amorphous aggregates | Organ dysfunction | Skin: bullous skin disease, papules, Schnitzler’s syndrome |
GI: diarrhea, malabsorption, bleeding | ||
Kidney: proteinuria, renal failure (light chain component) | ||
Tissue deposition as amyloid fibrils (light chain component most commonly) | Organ dysfunction | Fatigue, weight loss, edema, hepatomegaly, macroglossia, organ dysfunction of involved organs: heart, kidney, liver, peripheral sensory, and autonomic nerves |
16.4.1 Morbidity Mediated by the Physicochemical Properties of IgM
16.4.1.1 Hyperviscosity Syndrome
Blood hyperviscosity is the most distinguished feature of WM but is only observed in less than 15 % of patients at diagnosis, effected by increased serum IgM levels leading to hyperviscosity-related complications (Gertz and Kyle 1995). The mechanisms behind the marked increase in the resistance to blood flow and the resulting impaired transit through the microcirculatory system are rather complex (Gertz and Kyle 1995; Mackenzie and Babcock 1975; Kwaan and Bongu 1999). The main determinants are (1) a high concentration of monoclonal IgMs, which may form aggregates and may bind water through their carbohydrate component, and (2) their interaction with blood cells. Monoclonal IgMs increase red cell aggregation (rouleaux formation) and red cell internal viscosity while also reducing deformability. The possible presence of cryoglobulins can contribute to increasing blood viscosity as well as to the tendency to induce erythrocyte aggregation. Plasma viscosity and hematocrit are directly regulated by the body. Increased plasma viscosity may also contribute to inappropriately low erythropoietin production, which is the major reason for anemia in these patients (Singh et al. 1993). Clinical manifestations are related to circulatory disturbances that can be best appreciated by ophthalmoscopy, which shows distended and tortuous retinal veins, exudates such as cotton-wool spots, hemorrhages, and papilledema (Menke et al. 2006) (Fig. 16.1). Symptoms usually occur when the monoclonal IgM concentration exceeds 50 g/L or when serum viscosity is >4.0 centipoises (cp) (corresponding to a serum IgM level of at least 30 g/L), but there is a great individual variability, with some patients showing no evidence of hyperviscosity even at 10 cp (Mackenzie and Babcock 1975). The most common symptoms are oronasal bleeding, visual disturbances due to retinal bleeding, and dizziness that may rarely lead to coma. Heart failure can be aggravated, particularly in the elderly, owing to increased blood viscosity, expanded plasma volume, and anemia. Inappropriate transfusion can exacerbate hyperviscosity and may precipitate cardiac failure. Red cell transfusions should therefore be used with caution and sometimes in conjunction with pretransfusion plasmapheresis.
16.4.1.2 Type I Cryoglobulinemia
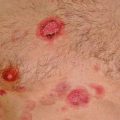
In up to 20 % of patients, monoclonal IgM may have tendency to precipitate upon cooling and, can thus behave as a type I cryoglobulin, but it is symptomatic in 5 % or less of the cases (Merlini et al. 2003). Cryoprecipitation is mainly dependent on the concentration of monoclonal IgM; for this reason, plasmapheresis or plasma exchange is commonly effective in this condition. Symptoms result from impaired blood flow in small vessels and include Raynaud’s phenomenon, acrocyanosis, and necrosis of the regions most exposed to cold such as the tip of the nose, ears, fingers, and toes (Fig. 16.2), malleolar ulcers, purpura, and cold urticaria. Renal manifestations may occur but are infrequent.
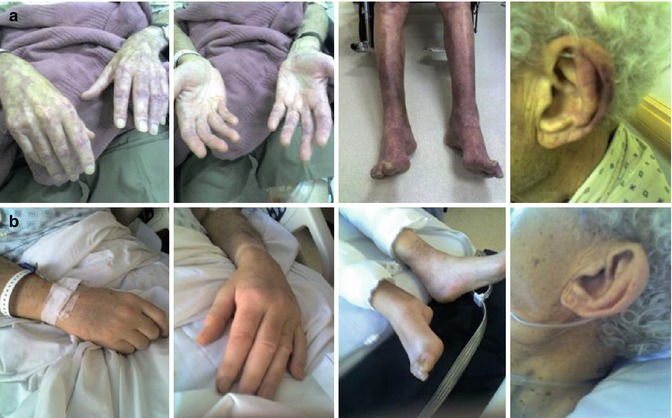

Fig. 16.2
Cryoglobulinemia manifesting with severe acrocyanosis in a patient with Waldenström’s macroglobulinemia before (a) and following warming and plasmapheresis (b)
16.4.1.3 Tissue Deposition
The monoclonal protein can deposit in several tissues as amorphous aggregates. Linear deposition of monoclonal IgM along the skin basement membrane is associated with bullous skin disease (Whittaker et al. 1996). Amorphous IgM deposits in the dermis determine the so-called IgM storage papules on the extensor surface of the extremities – macroglobulinemia cutis (Daoud et al. 1999). Deposition of monoclonal IgM in the lamina propria and/or submucosa of the intestine may be associated with diarrhea, malabsorption, and gastrointestinal bleeding (Gad et al. 1995; Case records of the Massachusetts General Hospital 1990). It is well known that kidney involvement is less common and less severe in WM than in multiple myeloma, probably because the amount of light chain excreted in the urine is generally lower in WM than in myeloma and because of the absence of contributing factors, such as hypercalcemia, although cast nephropathy has also been described in WM (Isaac and Herrera 2002). On the other hand, the IgM macromolecule is more susceptible to being trapped in the glomerular loops where ultrafiltration presumably contributes to its precipitation, forming subendothelial deposits of aggregated IgM proteins that occlude the glomerular capillaries (Morel-Maroger et al. 1970). Mild and reversible proteinuria may result, and most patients are asymptomatic. The deposition of monoclonal light chain as fibrillar amyloid deposits (AL amyloidosis) is uncommon in patients with WM (Gertz et al. 1993). In a large series of patients from the Mayo Clinic, amyloidosis develops in 2 % of patients with monoclonal IgM, among those 21 % had WM. Clinical expression and prognosis are similar to those of other AL patients with involvement of heart (44 %), kidneys (32 %), liver (14 %), lungs (10 %), peripheral/autonomic nerves (38 %), and soft tissues (18 %). In a French series of 72 patients, a peculiar pattern of relatively frequent lymph node (31 %) and lung (17 %) involvement was noted in patients with systemic AL amyloidosis (Terrier et al. 2008).
16.4.1.4 Interaction with Circulating Proteins
Monoclonal protein can interact with circulating proteins, including several coagulation factors, mainly factor VIII Willebrand and fibrinogen, and may cause prolonged clotting times. The macroglobulin can coat platelets, may impair their adhesion and aggregation, and may result in prolonged bleeding time (Farhangi and Merlini 1986).
16.4.2 Morbidity Mediated by the Immunological Effects of IgM
16.4.2.1 Autoantibody Activity
Monoclonal IgM may exert its pathogenic effects through specific recognition of autologous antigens, the most notable being nerve constituents, immunoglobulin determinants, and red blood cell antigens (reviewed in Stone and Pascual 2010).
16.4.2.2 Type II Cryoglobulinemia
In type II or mixed cryoglobulins, monoclonal IgM is an autoantibody to the Fc portion of polyclonal IgG. They are rheumatoid factor positive and often present at a high titer. The cryoprecipitating phenomenon is caused by the immune complex, as separation of the reactants yields clear solution. The manifestations are the same as previously described in type I. Renal manifestation particularly proliferative glomerulonephritis can be observed. Hepatitis C infection must be researched (Stone et al. 2005).
16.4.2.3 IgM-Related Neuropathy
The presence of peripheral neuropathy has been estimated to range from 5 to 38 % in WM patients (Dellagi et al. 1983; Nobile-Orazio et al. 1987; Nemni et al. 1994; Ropper and Gorson 1998; Treon et al. 2010). The nerve damage is mediated by diverse pathogenetic mechanisms: IgM antibody activity toward nerve constituents causing demyelinating polyneuropathies; endoneurial granulofibrillar deposits of IgM without antibody activity, associated with axonal polyneuropathy; and occasionally by tubular deposits in the endoneurium associated with IgM cryoglobulin and, rarely, by amyloid deposits or by neoplastic cell infiltration of nerve structures (Vital 2001). Half of the patients with IgM neuropathy have a distinctive clinical syndrome that is associated with antibodies against a minor 100-kDa glycoprotein component of nerve, myelin-associated glycoprotein (MAG). Anti-MAG antibodies are generally monoclonal IgMκ and usually also exhibit reactivity with other glycoproteins or glycolipids that share antigenic determinants with MAG (Latov et al. 1981; Chassande et al. 1998; Weiss et al. 1999). The anti-MAG-related neuropathy is typically distal and symmetrical, affecting both motor and sensory functions; it is slowly progressive with a long period of stability (Nobile-Orazio et al. 1987; Latov et al. 1988). Most patients present with sensory complaints (paresthesias, aching discomfort, dysesthesias, or lancinating pains), imbalance and gait ataxia, owing to lack proprioception, and leg muscles atrophy in advanced stage. Patients with predominantly demyelinating sensory neuropathy in association with monoclonal IgM to gangliosides with disialosyl moieties, such as GD1b, GD3, GD2, GT1b, and GQ1b, have also been reported (Dalakas and Quarles 1996; Eurelings et al. 2001). Anti-GD1b and anti-GQ1b antibodies were significantly associated with predominantly sensory ataxic neuropathy. These antiganglioside monoclonal IgMs present core clinical features of chronic ataxic neuropathy with variably present ophthalmoplegia and/or red blood cell cold agglutinating activity (CANOMAD). The disialosyl epitope is also present on red blood cell glycophorins, thereby accounting for the red cell cold agglutinin activity of anti-Pr2 specificity (Ilyas et al. 1985; Willison et al. 2001). Monoclonal IgM proteins that bind to gangliosides with a terminal trisaccharide moiety, including GM2 and GalNac-GD1A, are associated with chronic demyelinating neuropathy and severe sensory ataxia, unresponsive to corticosteroids (Lopate et al. 2002). Antiganglioside IgM proteins may also cross-react with lipopolysaccharides of Campylobacter jejuni, whose infection is known to precipitate the Miller Fisher syndrome, a variant of the Guillain–Barré syndrome (Jacobs et al. 1997). This finding indicates that molecular mimicry may play a role in this condition. Antisulfatide monoclonal IgM proteins, associated with sensory/sensorimotor neuropathy, have been detected in 5 % of patients with IgM monoclonal gammopathy and neuropathy (Nobile-Orazio et al. 1994). Motor neuron disease has been reported in patients with WM and monoclonal IgM with anti-GM1 and sulfoglucuronyl paragloboside activity (Gordon et al. 1997).
However, neuropathy in Waldenström’s macroglobulinemia (WM) is very heterogeneous. Neuropathy can be related to specific properties of the circulating IgM, leading to cryoglobulinemic or amyloid neuropathy or to neuropathy with endoneurial IgM deposits (Dellagi et al. 1983; Dimopoulos et al. 2000; Baehring et al. 2008). Neuropathy associated with tumoral infiltration, though rare, has also been described (Vital et al. 1982). For the neurologist and hematologist, diagnosing WM neuropathies is challenging because of their heterogeneous presentation. Yet it is crucially important to identify the mechanism involved in order to adapt the therapeutic strategy (Viala et al. 2012).
16.4.2.4 Cold Agglutinin Hemolytic Anemia
Monoclonal IgM may present with cold agglutinin activity, i.e., it can recognize specific red cell antigens at temperatures below physiological, producing chronic hemolytic anemia. This disorder occurs in <10 % of WM patients (Crisp and Pruzanski 1982) and is associated with cold agglutinin titers >1:1000 in most cases. The monoclonal component is usually an IgMκ and reacts most commonly with I/i antigens, with complement fixation and activation (Pruzanski and Shumak 1977a, b). The VH4-21 gene segment is necessary to encode anti-I specificity (Pascual et al. 1992). Many cold agglutinins have a high thermal amplitude so agglutination occurs in the 30–35 °C range. Mild chronic hemolytic anemia can be exacerbated after cold exposure but rarely does hemoglobin drop below 70 g/L. The hemolysis is usually extravascular (removal of C3b opsonized cells by the reticuloendothelial system, primarily in the liver) and rarely intravascular from complement destruction of red blood cell (RBC) membrane. The agglutination of RBCs in the cooler peripheral circulation also causes Raynaud’s syndrome, acrocyanosis, and livedo reticularis. Macroglobulins with the properties of both cryoglobulins and cold agglutinins with anti-Pr specificity have been reported. These properties may have as a common basis the immune binding of the sialic acid-containing carbohydrate present on red blood cell glycophorins and on Ig molecules. Several other macroglobulins with various antibody activities toward autologous antigens (i.e., phospholipids, tissue and plasma proteins) and foreign ligands have also been reported.
16.4.3 Manifestations Related to Tissue Infiltration by Neoplastic Cells
Tissue infiltration by neoplastic cells is rare and can involve various organs and tissues, from the bone marrow to the liver, spleen, lymph nodes, and possibly the lungs, gastrointestinal tract, kidneys, skin, eyes, and central nervous system. Pulmonary involvement in the form of masses, nodules, diffuse infiltrate, or pleural effusions is relatively rare, since the overall incidence of pulmonary and pleural findings reported for WM is only 3–5 % (Rausch and Herion 1980; Fadil and Taylor 1998; Kyrtsonis et al. 2001). Malabsorption, diarrhea, bleeding, or obstruction may indicate involvement of the gastrointestinal tract at the level of the stomach, duodenum, or small intestine (Kaila et al. 1996; Yasui et al. 1997; Rosenthal et al. 1998; Recine et al. 2001). The skin can be the site of dense lymphoplasmacytic infiltrates, similar to that seen in the liver, spleen, and lymph nodes, forming cutaneous plaques and, rarely, nodules (Mascaro et al. 1982). Chronic urticaria and IgM gammopathy are the two cardinal features of the Schnitzler syndrome, which is not usually associated initially with clinical features of WM (Schnitzler et al. 1974), although evolution to WM is not uncommon. Thus, close follow-up of these patients is warranted. Invasion of articular and periarticular structures by WM malignant cells is rarely reported (Roux et al. 1996). The neoplastic cells can infiltrate the periorbital structures, lacrimal gland, and retro-orbital lymphoid tissues, resulting in ocular nerve palsies (Orellana and Friedman 1981; Ettl et al. 1992). Direct infiltration of the central nervous system by monoclonal lymphoplasmacytic cells as infiltrates or as tumors constitutes the rarely observed Bing–Neel syndrome, characterized clinically by confusion, memory loss, disorientation, and motor dysfunction (reviewed in Malkani et al. 2010).
16.5 Laboratory Investigations and Findings
16.5.1 Hematological Abnormalities
Anemia is the most common finding in patients with symptomatic WM and is caused by a combination of factors: mild decrease in red cell survival, impaired erythropoiesis, hemolysis, moderate plasma volume expansion, and blood loss from the gastrointestinal tract. Blood smears are usually normocytic and normochromic, and rouleaux formation is often pronounced. Electronically measured mean corpuscular volume may be elevated spuriously owing to erythrocyte aggregation. In addition, the hemoglobin estimate can be inaccurate, i.e., falsely high, because of interaction between the monoclonal protein and the diluent used in some automated analyzers (McMullin et al. 1995). Leukocyte and platelet counts are usually within the reference range at presentation, although patients may occasionally present with severe thrombocytopenia. Monoclonal B-lymphocytes expressing surface IgM and late-differentiation B-cell markers are uncommonly detected in blood by flow cytometry. A raised erythrocyte sedimentation rate is almost constantly observed in WM and may be the first clue to the presence of the macroglobulin. The clotting abnormality detected most frequently is prolongation of thrombin time.
16.5.2 Biochemical Investigations
High-resolution electrophoresis combined with immunofixation of serum and urine is recommended for identification and characterization of the IgM monoclonal protein. The light chain of the monoclonal IgM is κ in 75–80 % of patients. A few WM patients have more than one M-component. The concentration of the serum monoclonal protein is very variable but in most cases lies within the range of 15–45 g/L. Densitometry should be adopted to determine IgM levels for serial evaluations because nephelometry is unreliable and shows large intralaboratory as well as interlaboratory variation. The presence of cold agglutinins or cryoglobulins may affect determination of IgM levels, and, therefore, testing for cold agglutinins and cryoglobulins should be performed at diagnosis. If present, subsequent serum samples should be analyzed under warm conditions for determination of serum monoclonal IgM level. Although Bence Jones proteinuria is frequently present, it exceeds 1 g/24 h in only 3 % of cases. While IgM levels are elevated in WM patients, IgA and IgG levels are most often depressed and do not demonstrate recovery even after successful treatment suggesting that patients with WM harbor a defect which prevents normal plasma cell development and/or Ig heavy chain rearrangements (Hunter et al. 2010; Treon et al. 2008a).
16.5.3 Serum Viscosity
Because of its large size (almost 1,000,000 Da), most IgM molecules are retained within the intravascular compartment and can exert an undue effect on serum viscosity. Therefore, serum viscosity should be measured if the patient has signs or symptoms of hyperviscosity syndrome. Fundoscopy remains an excellent indicator of clinically relevant hyperviscosity. Among the first clinical signs of hyperviscosity, the appearance of peripheral and mid-peripheral dot and blot-like hemorrhages in the retina, which are best appreciated with indirect ophthalmoscopy and scleral depression (Menke et al. 2006). In more severe cases of hyperviscosity, dot-, blot-, and flame-shaped hemorrhages can appear in the macular area along with markedly dilated and tortuous veins with focal constrictions resulting in “venous sausaging,” as well as papilledema.
16.6 Prognosis
Waldenström’s macroglobulinemia typically presents as an indolent disease though considerable variability in prognosis can be seen. The median survival reported in several large series has ranged from 5 to 10 years (Morel et al. 2000, 2009; Gobbi et al. 1994; Dhodapkar et al. 2001; Kyle et al. 2003; Dimopoulos et al. 2004; Anagnostopoulos et al. 2006a). Most studies have focused on overall survival from diagnosis to last follow-up, but others have analyzed survival after initiation of treatment in patients with symptomatic WM (Gobbi et al. 1994; Dhodapkar et al. 2001). Indeed, a high proportion of patients die from unrelated causes, because of their advanced age at diagnosis (Morel et al. 2000; García-Sanz et al. 2001; Gobbi et al. 1994). As previously underlines in epidemiology section, some series have shown a high incidence of cancer. The vital prognostic value of events during follow-up is unknown. Preliminary results pointed to the high incidence of long-lasting monoclonal component during the course of WM and the low frequency (6 %) of patients who experienced a rapid rise of the monoclonal component (Stalnikiewicz et al. 2003). These results suggested a heterogeneous disease course.
Age is consistently an important prognostic factor (>60–70 years) (Morel et al. 2000; Gobbi et al. 1994; Kyle et al. 2003; Morel et al. 2009), though is often impacted by unrelated morbidities. Anemia, which can be multifactorial, is an adverse prognostic factor in WM, with hemoglobin levels of <9–12 g/dL associated with decreased survival in several series (Morel et al. 2000, 2009; Gobbi et al. 1994; Dhodapkar et al. 2001). Cytopenias have also been regularly identified as a significant predictor of survival. The number of cytopenias in a given patient may predict survival (Morel et al. 2000). Serum albumin levels have correlated with survival in WM patients in certain but not all studies using multivariate analyses (Morel et al. 2000; Dimopoulos et al. 2004). High serum beta-2 microglobulin (>3–3.5 g/dL) levels (Dhodapkar et al. 2001; Dimopoulos et al. 2004; Morel et al. 2009), high serum IgM M-protein (>7 g/dL) (Morel et al. 2009), low serum IgM M-protein (<4 g/dL) (Dimopoulos et al. 2004), the presence of cryoglobulins (Gobbi et al. 1994), and the presence of a familial disease background (Treon 2011) have also been reported to confer adverse outcomes. The presence of 6q deletion as an adverse marker remains controversial (Ocio et al. 2007; Nguyen-Khac et al. 2013; Chang et al. 2009). A few prognostic scoring systems have been proposed, and the International Prognostic Scoring System is the most validated (Table 16.2).
Table 16.2
Prognostic scoring systems in Waldenström’s macroglobulinemia
Study | Adverse prognostic factors | Number of groups | Survival |
|---|---|---|---|
Gobbi et al. (1994) | Hb < 9 g/dL | 0–1 prognostic factors | Median: 48 month |
Age >70 year | 2–4 prognostic factors | Median: 80 month | |
Weight loss | |||
Cryoglobulinemia | |||
Morel et al. (2000) | Age ≥ 65 year | 0–1 prognostic factors | 5 year: 87 % |
Albumin < 4 g/dL | 2 prognostic factors | 5 year: 62 % | |
Number of cytopenias: | 3–4 prognostic factors | 5 year: 25 % | |
Hb < 12 g/dL | |||
Platelets < 150 × 109/L | |||
Wbc < 4 × 109/L | |||
Dhodapkar et al. (2001) | β2M ≥ 3 g/dL | β2M < 3 mg/dL + Hb ≥ 12 g/dL | 5 year: 87 % |
Hb < 12 g/dL | β2M < 3 mg/dL + Hb < 12 g/dL | 5 year: 63 % | |
IgM < 4 g/dL | β2M ≥ 3 mg/dL + IgM ≥ 4 g/dL | 5 year: 53 % | |
β2M ≥ 3 mg/dL + IgM < 4 g/dL | 5 year: 21 % | ||
Application of International Staging System Criteria for Myeloma to WM Dimopoulos et al. (2004) | Albumin ≤3.5 g/dL | Albumin ≥ 3.5 g/dL + β2M < 3.5 mg/dL | Median: NR |
β2M ≥ 3.5 mg/L | Albumin ≤ 3.5 g/dL + β2M < 3.5 or β2M 3.5–5.5 mg/dL | Median: 116 month | |
β2M > 5.5 mg/dL | Median: 54 month | ||
International Prognostic Scoring System for WM Morel et al. (2009) | Age > 65 year | 0–1 prognostic factors* | 5 year: 87 % |
Hb < 11.5 g/dL | 2 prognostic factors** | 5 year: 68 % | |
Platelets < 100 × 109/L | 3–5 prognostic factors | 5 year: 36 % | |
β2M > 3 mg/L | *excluding age | ||
IgM > 7 g/dL | ** or age >65 |
16.7 Treatment of Waldenström’s Macroglobulinemia
16.7.1 Treatment Indications
Consensus guidelines on indications for treatment initiation were formulated as part of the 2nd International Workshop on Waldenström’s macroglobulinemia (Kyle et al. 2003). Initiation of therapy should not be based on the IgM levels since this may not correlate with either disease burden or symptomatic status (Treon and How 2009; Dimopoulos et al. 2009). Initiation of therapy is appropriate for patients with constitutional symptoms, such as recurrent fever, night sweats, fatigue due to anemia, or weight loss. The presence of progressive, symptomatic lymphadenopathy or splenomegaly provides additional reasons to begin therapy. The presence of anemia with a hemoglobin value of ≤10 g/dL or a platelet count ≤100 × 109/L on this basis of disease is also a reasonable indication for treatment initiation. Certain complications of WM, such as hyperviscosity syndrome, symptomatic sensorimotor peripheral neuropathy, systemic amyloidosis, renal insufficiency, or symptomatic cryoglobulinemia, are also indications for therapy.
16.7.2 Treatment Options
A precise therapeutic algorithm for therapy of WM remains to be defined given the paucity of randomized clinical trials. Active agents include alkylators (chlorambucil, cyclophosphamide), nucleoside analogues (cladribine, fludarabine), monoclonal antibodies (rituximab, ofatumumab, alemtuzumab), bortezomib, thalidomide, everolimus, and bendamustine (Treon and How 2009; Dimopoulos et al. 2009). Combination therapy particularly with rituximab has been associated with improved clinical outcomes. Individual patient considerations, including the presence of cytopenias, need for more rapid disease control, age, and candidacy for autologous transplant therapy, should be taken into account in making the choice of a first-line agent. For patients who are candidates for autologous transplant therapy, exposure to continuous chlorambucil or nucleoside analogue therapy should be limited given potential for stem cell damage.
16.7.2.1 Plasmapheresis
Because 80 % of IgM is intravascular, plasmapheresis, conducted with a continuous blood flow separator with albumin and saline replacement, is very effective in reducing rapidly the amount of circulating IgM. Plasmapheresis is indicated for the treatment of patients who present with or develop symptomatic hyperviscosity. Even small reductions of serum IgM concentration with plasmapheresis can reduce significantly serum viscosity and can lead to resolution of hyperviscosity-related symptoms. Reductions of IgM by an average of 35 % resulted in a decrease of plasma viscosity from 5 to 2.1 (Kaplan 2001). In most patients with symptomatic hyperviscosity, concomitant administration of systemic treatment is required in order to suppress the underlying malignant process. However, some patients with predominant symptoms of hyperviscosity have been effectively managed for several years with plasmapheresis alone. This strategy may be also considered in patients who fail systemic treatment and who suffer primarily of hyperviscosity. Intensive plasmapheresis has also been used successfully in some patients with an IgM-related disorder such as peripheral neuropathy, cryoglobulinemia, and cold agglutinin disease. In such patients, a series of plasmapheresis may reduce the monoclonal protein, provide an opportunity for symptomatic improvement, and justify the subsequent administration of systemic therapy to achieve long-term control.
16.7.2.2 Chlorambucil
Oral alkylating drugs, alone and in combination therapy with steroids, have been extensively evaluated in the upfront treatment of WM. The greatest experience with oral alkylator therapy has been with chlorambucil, which has been administered on both a continuous (i.e., daily dose schedule) and an intermittent schedule. Kyle et al. (2000) reported no significant difference in the overall response rate between these schedules, although interestingly the median response duration was greater for patients receiving intermittent versus continuously dosed chlorambucil (46 vs. 26 months). Approximately 50 % will achieve a response, but complete responses are uncommon. The use of steroids in combination with alkylator therapy has also been explored and has not been shown to affect response rate or overall survival but may be of benefit when WM is associated with autoimmune phenomena (Dimopoulos and Alexanian 1994).
Non-chlorambucil-based alkylator regimens employing melphalan and cyclophosphamide in combination with steroids have also been examined by Petrucci et al. (1989) and Case et al. (1991) producing slightly higher overall response rates and response durations, although the benefit of these more complex regimens over chlorambucil remains to be demonstrated. Additional factors to be taken into account in considering alkylator therapy for patients with WM include necessity for more rapid disease control given the slow nature of response to alkylator therapy, as well as consideration for preserving stem cells in patients who are candidates for autologous transplant therapy.
In a randomized study comparing the efficacy of fludarabine to that of chlorambucil, the response rate of 171 patients treated with chlorambucil was 36 % and the relapse-free survival time was 21.3 months with a response duration of 34.6 months. A higher cumulative incidence of second malignancies with a 6-year cumulative incidence of 3.7 % in the fludarabine arm and 20.6 % in the chlorambucil arm (p = 0.001) was observed in patients treated with chlorambucil (Leblond et al. 2013).
16.7.2.3 Nucleoside Analogues
Both cladribine and fludarabine have been extensively evaluated in untreated as well as previously treated WM patients (Dimopoulos et al. 1993, 1994a, b, 1995; Delannoy et al. 1994; Fridrik et al. 1997; Liu et al. 1998; Hellmann et al. 1999; Betticher et al. 1997; Foran et al. 1999; Thalhammer-Scherrer et al. 2000; Zinzani et al. 1995; Leblond et al. 1998, 2001; Lewandowski et al. 2002). Cladribine administered as a single agent by continuous intravenous infusion, by 2-h daily infusion, or by subcutaneous bolus injections for 5–7 days has resulted in major responses in 40–90 % of patients who received primary therapy, while in the salvage setting responses have ranged from 38 to 54 % (Dimopoulos et al. 1994a, 1995; Delannoy et al. 1994; Fridrik et al. 1997; Liu et al. 1998; Hellmann et al. 1999; Betticher et al. 1997). Median time for achievement of response following cladribine ranged from 1.2 to 5 months in these studies. The overall response rate with daily infusional fludarabine therapy administered mainly on 5-day schedules in previously untreated and treated WM patients has ranged from 38 to 100 % and 30 to 40 %, respectively (Dhodapkar et al. 2001; Dimopoulos et al. 1993; Foran et al. 1999; Thalhammer-Scherrer et al. 2000; Zinzani et al. 1995; Leblond et al. 1998, 2001), which are on par with the response data for cladribine. In a large randomized study in 168 untreated patients, the fludarabine response rate was 46 %, the relapse-free survival time 38.5 months, and the response duration was 50.1 months (Leblond et al. 2013).
Median time to achievement of response for fludarabine was also on par with cladribine at 3–6 months but took more than 6 months and more than 1 year in respectively 17 % and 5 % of responders in a large phase II study (Dhodapkar et al. 2001). In general, response rates and durations of responses have been greater for patients receiving nucleoside analogues as first-line agents.
Purine analogues (both fludarabine and 2 CDA) are effective in patients who are primary resistant or relapse after alkylating agents. Several phase II studies of purine analogues have involved patients who had received prior therapy (usually alkylating agents). The response rates varied from 14 to 78 %. Fludarabine induces responses in about one-third of patients who were resistant to a previous treatment and is highest in patients who are still sensitive to their primary therapy (Dimopoulos et al. 1993; Leblond et al. 2001).
Myelosuppression commonly occurred following prolonged exposure to either of the nucleoside analogues, as did lymphopenia with sustained depletion of both CD4+ and CD8+ T-lymphocytes observed in WM patients 1 year following initiation of therapy. Treatment-related mortality due to myelosuppression and/or opportunistic infections attributable to immunosuppression occurred in up to 5 % of all treated patients in some series with either nucleoside analogue. The combination of nucleoside analogues with cyclophosphamide and/or rituximab has been investigated and discussed below.
The safety of nucleoside analogues has been the subject of investigation in several recent studies. The principal toxicity of purine analogues is myelosuppression. For patients in whom high-dose chemotherapy and autologous stem cell transplantation are being considered, nucleoside analogues must be used with precaution, as several published data have shown that stem cell collection can be unsuccessful after fludarabine-containing regimens. The use of stem cell-damaging agents thus has to be reconsidered when the therapeutic strategy includes high-dose therapy and autologous stem cell transplantation (Thomas et al. 2008). The long-term safety of nucleoside analogues in WM was examined by Leleu et al. (2009a) in a large series of WM patients. A sevenfold increase in transformation to an aggressive lymphoma and a threefold increase in the development of acute myelogenous leukemia/myelodysplasia were observed among patients who received a nucleoside analogue versus other therapies for their WM. A meta-analysis by Leleu et al. (2009b) of several trials utilizing nucleoside analogues in WM patients, which included patients who had previously received an alkylator agent, showed a crude incidence of 6.6–10 % for development of disease transformation and 1.4–8.9 % for development of myelodysplasia or acute myelogenous leukemia. These results were not confirmed in a large randomized study comparing the efficacy of fludarabine alone to that of chlorambucil with a 6-year cumulative incidence of disease transformation of 7.7 % in the fludarabine arm versus 11.1 % in the chlorambucil arm. Three MDS/AMLs were observed during the follow-up, all cases in the chlorambucil arm (Leblond et al. 2013). However, there is some evidence to suggest that this complication may be more frequent in patients treated fludarabine-alkylator combinations than with fludarabine monotherapy (Carney et al. 2010; Smith et al. 2011).
16.7.2.4 Monoclonal Antibodies
Rituximab is a chimeric monoclonal antibody which targets CD20, a widely expressed antigen on lymphoplasmacytic cells in WM (Treon et al. 2003). The use of rituximab at standard dosimetry (i.e., 4 weekly infusions at 375 mg/m2) induces major responses in approximately 27–35 % of previously treated and untreated patients (Treon et al. 2001; Gertz et al. 2004). However, patients who achieved even minor responses benefited from rituximab as evidenced by improved hemoglobin and platelet counts and reduction of lymphadenopathy and/or splenomegaly (Gertz et al. 2004). The median time to treatment failure in these studies was found to range from 8 to 27+ months. Studies evaluating an extended rituximab schedule consisting of 4 weekly courses at 375 mg/m2/week, repeated 3 months later by another 4 week course, have demonstrated higher major response rates of 44–48 %, with time to progression estimates of 16+ to 29+ months (Dimopoulos et al. 2002; Treon et al. 2005a).
In many WM patients, a transient increase of serum IgM (IgM flare) may be noted immediately following initiation of rituximab treatment (Donnelly et al. 2001; Treon et al. 2004; Ghobrial et al. 2004). The IgM flare may be related to release of interleukin-6 by bystander immune in response to binding of rituximab to FcγRIIA receptors and also occurs in response to intravenous immunoglobulin administration in WM patients (Yang et al. 2010). The IgM flare in response to rituximab does not herald treatment failure, and while most patients will return to their baseline serum IgM level by 12 weeks, some patients may flare for months despite having tumor responses in their bone marrow. Patients with baseline serum IgM levels of >50 g/dL or serum viscosity of >3.5 cp may be particularly at risk for a hyperviscosity-related event, and in such patients plasmapheresis should be considered or rituximab omitted for the first few cycles of therapy until IgM levels decline to safer levels. Because of the decreased likelihood of response in patients with higher IgM levels, as well as the possibility that serum IgM and viscosity levels may abruptly rise, rituximab monotherapy should not be used as sole therapy for the treatment of patients at risk for hyperviscosity symptoms.
Time to response after rituximab is slow and exceeds 3 months on the average. The time to best response in one study was 18 months (Treon et al. 2005a). Patients with baseline serum IgM levels of <60 g/dL are more likely to respond, irrespective of the underlying bone marrow involvement by tumor cells (Dimopoulos et al. 2002; Treon et al. 2005a). An analysis of 52 patients who were treated with single-agent rituximab has indicated that the objective response rate was significantly lower in patients who had either low serum albumin (<35 g/L) or elevated serum monoclonal protein (>40 g/L M-spike). Furthermore, the presence of both adverse prognostic factors was related with a short time to progression (3.6 months). Moreover patients who had normal serum albumin and relatively low serum monoclonal protein levels derived a substantial benefit from rituximab with a time to progression exceeding 40 months (Dimopoulos et al. 2005a).
The genetic background of patients may also be important for determining response to rituximab. A correlation between polymorphisms at amino acid position 158 in the Fc gamma RIIIa receptor (CD16) and rituximab response has been observed in WM patients. WM patients who carry a valine amino acid (either in a homozygous or heterozygous pattern) at this polymorphic site had a fourfold higher major response rate to rituximab versus patients who expressed phenylalanine in a homozygous pattern (Treon et al. 2005b). The attainment of better categorical responses, i.e., very good partial response or complete response following rituximab-based therapy, appears also dependent on the presence of at least one valine amino acid at FcγRIIIa-158 (Treon et al. 2011a).
Ofatumumab is a fully humanized CD20-directed monoclonal antibody that targets the small loop of CD20, a target which is different than that of rituximab. A 59 % overall response rate was observed in a series of 37 symptomatic WM patients following ofatumumab administration, which included untreated and previously treated patients (Furman et al. 2011). Responses were higher among rituximab-naïve patients. An IgM flare with symptomatic hyperviscosity was also observed in two patients in this series who required plasmapheresis.
The activity of alemtuzumab has also been investigated in WM patients given the broad expression of CD52 (Treon et al. 2003). The WMCTG recently reported a multicenter study in symptomatic WM patients, whose median prior therapies was 2 (range 0–5), and 43 % had refractory disease (Treon et al. 2011b). Patients received alemtuzumab intravenously at 30 mg three times weekly for up to 12 weeks, after test dosing, and received hydrocortisone, acyclovir, and Bactrim or equivalent prophylaxis. The overall response rate in this series was 75 % and included major responses in 36 % of patients. With a median follow-up of 64 months, the median time to progression was 14.5 months. Hematological and infectious complications, including CMV reactivation, were more common in previously treated patients and indirectly associated with three deaths. Long-term follow-up revealed late-onset idiopathic thrombocytopenia in four patients at a median of 13.6 months following therapy and contributed to one death. High rates of response with the use of alemtuzumab were also observed by Owen et al. (2003b) who reported their preliminary experience in a small series of heavily pretreated WM patients.
16.7.2.5 Bortezomib
Bortezomib is a proteasome inhibitor which has been extensively investigated in WM. In a multicenter study of the WMCTG, 27 patients received up to 8 cycles of bortezomib at 1.3 mg/m2 on days 1, 4, 8, and 11 (Treon et al. 2007). All but one patient had relapsed/or refractory disease. The overall response rate was 85 %, with 10 and 13 patients achieving a minor (<25 % decrease in IgM) and major (<50 % decrease in IgM) response. Responses were prompt and occurred at median of 1.4 months. The median time to progression for all responding patients in this study was 7.9 (range 3–21.4+) months, and the most common grade III/IV toxicities occurring in ≥5 % of patients were sensory neuropathies (22.2 %), leukopenia (18.5 %), neutropenia (14.8 %), dizziness (11.1 %), and thrombocytopenia (7.4 %). Importantly, sensory neuropathies resolved or improved in nearly all patients following cessation of therapy. As part of an NCI-Canada study, Chen et al. (2007) treated 27 patients with both untreated (44 %) and previously treated (56 %) disease. Patients in this study received bortezomib utilizing the standard schedule until they either demonstrated progressive disease or two cycles beyond a complete response or stable disease. The overall response rate in this study was 78 %, with major responses observed in 44 % of patients. Sensory neuropathy occurred in 20 patients, 5 with grade >3, and occurred following 2–4 cycles of therapy. Among the 20 patients developing a neuropathy, 14 patients resolved and one patient demonstrated a one-grade improvement at 2–13 months. In addition to the above experiences with bortezomib monotherapy in WM, Dimopoulos et al. (2005b) observed major responses in 6 of 10 (60 %) previously treated WM patients. The combination of bortezomib with steroids and/or rituximab has also been investigated and is discussed below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree