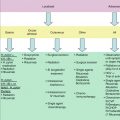
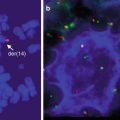
Fig. 17.1
Blood smear of a B-cell prolymphocytic leukemia. An 84-year-old man presented with abdominal pain to the emergency room with a WBC of 140,000 and splenomegaly. There is marked lymphocytosis with a population of medium-sized lymphocytes with nucleoli. FISH and immunohistochemistry (on bone marrow) for cyclin D1 was negative
17.2 Pathology of B-PLL and T-PLL
In B-PLL, in the peripheral blood, >55 % of the lymphocytes are prolymphocytes. They have a small rim of slightly basophilic cytoplasm, round even nuclei with a moderately condensed cytoplasm, and, usually, a single central nucleolus. T-PLL cells are medium-sized lymphocytes with deeply basophilic cytoplasm without granules and oval or irregular nuclei with deep indentations (Pawson et al. 1997). In the bone marrow, the infiltration pattern of both B-PLL and T-PLL may be interstitial, nodular, diffuse, or patchy. In the spleen, the white pulp nodules are enlarged in B-PLL, often producing a multinodular, at times coalescing, architecture. Red pulp sinusoids are variably expanded by prolymphocytes (Galton et al. 1974; Lampert et al. 1980; Ruchlemer et al. 2004). In T-PLL, the infiltration is mainly found in the red pulp (Osuji et al. 2005). Lymph nodes in B-PLL may show – rarely – patchy or vaguely nodular or diffuse infiltrates. In those cases, the picture may be similar to cases of prolymphocyte-rich B-CLL, but pseudofollicles are absent. In contrast, in T-PLL, the infiltration of lymph nodes is frequent, and infiltrates are centered in the interfollicular area, sometimes sparing residual germinal centers. One characteristic feature of T-PLL is the presence of arborizing high-endothelial venules that are transmigrated by lymphoma cells. The liver may show portal and sinusoidal infiltrates in both B-PLL and T-PLL. Skin infiltrations in T-PLL are usually confined to the dermis (Matutes et al. 1991).
For the pathologist, the prolymphocytes in B-PLL, in tissue sections, may be more similar to the paraimmunoblast as defined in the Kiel classification of lymphomas (Lennert 1992), and hence, differentiation from progressed (paraimmunoblast-rich or prolymphocyte-rich) stages of B-CLL may prove difficult. However, in contrast to B-CLL and B-CLL/PL, proliferation centers are absent. Presence of the t(11;14)(q13;q32) chromosomal translocation excludes the diagnosis of B-PLL (Dunphy and Perkins 2001; Ruchlemer et al. 2004; Singleton et al. 1999).
17.3 Immunophenotype of B-PLL and T-PLL
Prolymphocytes in B-PLL express pan-B-cell-associated antigens CD19, CD20, CD22, CD79A, PAX5, and FMC7 and strongly express surface IgM ± IgD. Roughly 50 % of cases will show positivity for CD5, while CD23 is rarely expressed. CyclinD1, CD10, and BCL6 are negative (Campo et al. 2008; Hercher et al. 2001). T-PLL cells express pan-T-cell markers such as CD3, CD2, CD5, and CD7, and most cases are CD4+. However, both CD4- CD8+ and CD4+ CD8+ cases exist. TCRαβ and TCL1 proteins are usually positive, and NK markers are not expressed (Matutes et al. 1991).
17.4 Molecular Genetics of B-PLL and T-PLL
Immunoglobulin heavy chain (IGH) genes in B-PLL are clonally rearranged. B-PLL is a lymphoid neoplasia apparently deriving from both mutated and unmutated progenitor cells. Therefore, mutated IGHV genes are encountered in roughly 50 % of cases. Most B-PLL cases have been reported to use members of the VH3 and VH4 gene families, respectively (Davi et al. 1996; Del Giudice et al. 2006a, b). The t(11;14) chromosome translocation had been described in 20 % of cases that were previously thought to represent B-PLL owing to the prolymphocytic appearance of the tumor cells. To date, however, these lymphomas have been recognized as mantle cell lymphomas (MCL) with “prolymphocytic” features (Dunphy and Perkins 2001; Wong et al. 2002). Ninety of T-PLL cases usually demonstrate clonal chromosomal aberrations involving inversions of the TCL1 in 14q32 or MTCP1 in Xq28 loci with the TCRA/D locus in 14q11 (inv14q11q32) (Pekarsky et al. 1999; Stern et al. 1993). T-PLL is among those lymphoid tumors with the highest number of secondary chromosomal aberrations (Nowak et al. 2009), in virtually all sporadic cases also targeting the ATM gene in 11q21-q23 that usually shows biallelic inactivation by missense mutations (Stilgenbauer et al. 1997). Trisomies of chromosome 8 or 8q are also common.
17.5 Differential Diagnosis of B-PLL and T-PLL
The diagnosis of B-PLL warrants the exclusion of morphologically and clinically related entities, such as B-CLL with increased prolymphocytes, leukemic MCL, splenic marginal zone lymphoma (SMZL), hairy cell leukemia-variant (HCLv), and T-cell prolymphocytic leukemia (Viswanatha et al. 2012). In some cases, the distinction from “paraimmunoblastic” diffuse large B-cell lymphomas may equally prove difficult. While B-CLL, DLBCL, and T-PLL may be readily distinguished by their clinical presentation and/or immunophenotype, splenomegalic and leukemic forms of MCL have to be excluded by CyclinD1 staining and/or FISH for the t(11;14). The unequivocal exclusion of entities like SMZL and HCLv requires attention, because both the clinical presentation and the cytological presentation of the tumor cells may be similar. In general, however, the tumor cells in SMZL are more polymorphic with “villous” lymphocytes apparent, and HCLv is excluded by its distinct antigen expression profile including CD103 (Matutes et al. 2003).
The differentiation of T-PLL from other leukemic T-cell lymphomas may be challenging because of similarities in morphology and immunophenotype to other leukemic lymphomas such as hepatosplenic T-cell lymphoma, Sezary syndrome, or T-LGL. T-PLL, in contrast to the other entities, does show protein expression of TCL1 and CD26. Ultimately, however, the diagnosis must be confirmed by demonstration of the characteristic genetic profile of the disease including rearrangements of TCL1, MTCP1, trisomy 8 or 8q, and ATM inactivation.
17.6 Prognosis and Therapy of B-PLL
B-PLL initially described as a variant of CLL, is now recognized as a distinct clinicopathological entity in the current WHO classification, typically with an aggressive clinical course and a median overall survival of 3–4 years (Swerdlow et al. 2008). Historical series had included a substantial proportion of patients with t(11;14), but these are now classified as leukemic-phase MCL.
17.6.1 Conventional Therapy of B-PLL
The clinical course of B-PLL is usually aggressive; however, in some series, an initially indolent disease has been seen in up to 25 % of cases (Shvidel et al. 1999). Analogous to CLL, alkylation agents such as chlorambucil or even alkylating agent-based poly-chemotherapy, e.g., CHOP, have been used in B-PLL, but efficacy is only modest with response rates < 35 % (Sibbald and Catovsky 1979). Purine analogues such as fludarabine, cladribine, or pentostatin either alone or in combination with cyclophosphamide induce an overall response rate (ORR) of 35–50 %. However, the duration of response is usually less than 2 years (Herold et al. 2012; Rondelli et al. 1997; Saven et al. 1997).
Anecdotal series report on single-agent activity of the anti-CD20 monoclonal antibody rituximab (Mourad et al. 2004; Vartholomatos et al. 1999), and this observation has led to routine usage of combination chemoimmunotherapy, e.g., either fludarabine or bendamustine with an anthracycline (mitoxantrone or epirubicin) and rituximab in mixed populations including of CLL and B-PLL (Chow et al. 2011; Vartholomatos et al. 1999; Weide et al. 2004).
Although there is clear evidence for the benefit of adding rituximab to fludarabine-based chemotherapy in CLL (Hallek et al. 2010), there is no such data available in B-PLL. In order to better define the role of rituximab in combination with chemotherapy (e.g., fludarabine and cyclophosphamide (R-FC)), a prospective multicenter trial including only patients with chemotherapy naive B-PLL was started within the German CLL Study Group; however, recruitment was low, and therefore, the trial was stopped early. Complex cytogenetic aberrations are usually present in B-PLL, in particular about 50 % of patients with B-PLL display a TP53, which may explain the often refractory disease after conventional therapy (Dearden 2012; Lens et al. 2000; Put et al. 2012). There are also single cases reporting the activity of the anti-CDC52 antibody alemtuzumab in B-PLL (Bowen et al. 1997; Chaar and Petruska 2007; Dearden 2012; Lens et al. 2000; Put et al. 2012). Splenectomy is recommended only in a palliative setting but can provide good symptomatic relief (Matutes 2012). Central nervous system involvement has been reported and is usually a fatal event (Pamuk et al. 2009).
17.6.2 High-Dose Therapy of B-PLL
So far, again only case reports or small retrospective series are available, demonstrating substantial impact of either ASCT (Shvidel et al. 2000) or allogeneic transplant in selected B-PLL patients’ (Castagna et al. 2005) registry data of the International Bone Marrow Transplant Registry. Concerning the latter publication, however, it must be noted that B- and T-PLL were not described separately within the overall group of 47 patients. However, no difference was found with regard to B-cell versus T-cell PLL, neither was there found a difference in a 2-year overall survival (OS) between patients with either acute or chronic Graft-versus-Host-Disease (GVHD); median PFS was 3.5 months for the patients with B-PLL (n = 11) with ~30 % remaining alive and relapse-free beyond 12 months (Kalaycio et al. 2010).
17.7 Prognosis and Therapy of T-PLL
In most patients, T-PLL shows an initially aggressive course and often chemo-refractory behavior to conventional cytotoxic agents resulting early relapses within 1 year (Herling et al. 2004; Herling et al. 2008; Matutes 1998; Matutes et al. 1991).
Some anecdotal reports and small series have described apparently initially indolent cases of T-PLL either associated with a complex karyotype (Soma et al. 2002) or a specific immunophenotype with a CD45RO+/CD45RA- phenotype (Garand et al. 1998). Despite this initial indolent phase with a median of 33 months until requiring treatment, most patients then display an aggressive course and an outcome that is nearly as poor as for patients showing a primarily aggressive course (Garand et al. 1998).
17.7.1 Conventional Therapy of T-PLL
Typically, T-PLL shows a marked resistance to conventional chemotherapy, as only 30–45 % of patients will achieve a response when alkylating agents such as chlorambucil or poly-chemotherapy based on alkylating agents or regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or VAPEC-B (vincristine, doxorubicin, prednisone, etoposide, cyclophosphamide, bleomycin) and no complete remissions were reported (Dearden et al. 2001).
These disappointing response rates are somewhat improved by the use of purine nucleoside analogues such as fludarabine or in particular by pentostatin (2′-deoxycoformycin), a potent inhibitor of adenosine deaminase. Pentostatin is usually administered at a dosage of 4 mg/m2 i.v. every week leading to an ORR of 8–45 % including CR 9 % (Mercieca et al. 1994). Observed toxicity was usually low, but includes nausea/vomiting and modest hematologic toxicity.
More recently, response rates have been dramatically improved with the introduction of alemtuzumab, a humanized monoclonal antibody against the pan-lymphocyte antigen CD52, acting via cell death induction, complement activation, antibody-dependent cytotoxicity, and apoptosis (Dyer et al. 1989; Ginaldi et al. 1998; Greenwood et al. 1993; Heit et al. 1986; Rowan et al. 1998). So far, alemtuzumab is the most active single drug in T-PLL demonstrating response rates of 50–75 % in patients with relapsed or refractory disease.
Initially, alemtuzumab was investigated in a single-center retrospective analysis including 15 patients with relapsed T-PLL resulting in encouraging overall response of 73 % (CR 60 %). However, the median PFS was only 8 months (Pawson et al. 1997).
In a larger multicenter, prospective trial, 39 patients with relapsed T-PLL were treated with alemtuzumab resulting in an ORR of 76 % (CR 60 %) (Dearden et al. 2001). This impressive level of activity was confirmed when evaluating a compassionate use program with 79 patients with an ORR of 50 % (CR 38 %); however, long-term outcome again was poor, with a median PFS of 4.5 months (range, 0.1–45.4 months), and the median OS was only 7.5 months (Keating et al. 2002). Notably, the series of Keating et al. included four chemo-naive patients, with three who achieved a response.
However, given these encouraging response rates, alemtuzumab was then investigated as first-line therapy. In a recent report, alemtuzumab was administered to 32 patients using the i.v. route resulting in an OOR of 91 % including a very high CR rate of 81 %. Furthermore, this study emphasizes the importance of route of administration as in nine patients alemtuzumab was given subcutaneously resulting in an ORR of only 32 %, which compared unfavorably to the i.v. route (Dearden et al. 2011). A 12-month PFS rate of 67 % and an OS of 37 % at 48 months were described for the i.v. cohort. In analogy to other malignant lymphatic neoplasms, combination of monoclonal antibodies with chemotherapy would seem to be worth to be investigated, e.g., bendamustine, a drug widely used in non-Hodgkin lymphoma, recently showed activity in T-cell lymphoma alone and in T-PLL in combination with alemtuzumab (Yong et al. 2012).
However, in the clinical setting, a combination of alemtuzumab and pentostatin in 13 pretreated patients with T-PLL showed no further improvement compared with alemtuzumab alone (ORR 69 %, CR 62 %) (Ravandi et al. 2009). In a multicenter prospective trial of the German CLL Study Group, 25 patients (16 chemo-naive, 9 pretreated) received a fludarabine-based induction chemotherapy (FMC: fludarabine phosphate 25 mg/m2/day (i.v.) on days 1–3, mitoxantrone 8 mg/m2/day i.v. on day 1, and cyclophosphamide 200 mg/m2/d i.v. on days 1–3 to be repeated on day 28) followed by an alemtuzumab consolidation. After a run-in phase, alemtuzumab was administered 30 mg i.v. three times weekly as consolidation for a maximum of 12 weeks. Four of 25 patients received only FMC, and 21 received FMC followed by alemtuzumab. Hematologic toxicities were the most frequent grade 3/4 side effects seen with FMC-A. Neutropenia represented 10 % of the cumulative overall grade 3/4 events after FMC and 16 % after alemtuzumab. There were also 13 cases of CMV reactivation (62 % of patients) during the alemtuzumab phase. The ORR was 92 % (CR 46 %) which is similar to reports using alemtuzumab alone. However, the median PFS and OS were 11.5 and 17.1 months, respectively, suggesting benefit from the sequential therapy on long-term outcome although no plateau in survival was seen (Hopfinger et al. 2013). For an overview on conventional therapy, see Table 17.1.
Table 17.1
Published series on systematic treatment evaluations in T-PLL (Adopted from Hopfinger et al. 2013)
Reference | Regimen | Disease status | Trial details | n | CR (%) | PR (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
Mercieca et al. (1994) | Pentostatin | Pretreated | Single-center retrospective | 56 | 9 | 36 | 6 | 9 |
Pawson et al. (1997) | Alemtuzumab i.v. | Pretreated | Single-center retrospective | 15 | 60 | 13 | 6 | 8 |
Dearden et al. (2001) | Alemtuzumab i.v. | Pretreated | Multicenter prospective | 39 | 60 | 16 | 7 | 10 |
Keating et al. (2002) | Alemtuzumab i.v. | Pretreated (4 untreated) | Multicenter retrospective | 76 | 38 | 12 | 4.5 | 7.5 |
Ravandi et al. (2009) | Pentostatin + alemtuzumab i.v. | Pretreated | Single-center prospective | 13 | 62 | 8 | 7.8 | 10.2 |
Dearden et al. (2011) | Alemtuzumab i.v. | Untreated | Single-center | 32 | 81 | 10 | (67 %)a | (37 %)a |
Alemtuzumab s.c. | Untreated | prospective | 9 | 33 | 0 | (67 %)a | (33 %)a | |
Hopfinger et al. (2013) | FMC then alemtuzumab i.v. | 9 pretreated | Multicenter prospective | 25 | 46 | 46 | 11.5 | 17.1 |
16 untreated |
In conclusion, typically outcomes after second or salvage therapies are very poor, and it is extremely rare to attain durable responses to such conventional salvage therapy.
17.7.2 High-Dose Therapy of T-PLL
Several strategies have been pursued in order to improve the dismal outcome of T-PLL by introducing high-dose therapy followed by either autologous stem cell transplantation (ASCT) or allogeneic transplant in T-PLL; see Table 17.2.
Table 17.2
Published series on autologous/allogeneic transplant in T-PLL
Reference | Regimen | Disease status at Tx | Source | n | TRM (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
Collins et al. (1998) | Cy/TBI | PD | BM, sibling | 1 | – | 36 + | 36 + |
Garderet et al. (2001) | Bu/Cy/ATG (nonablative) | PR | BM, sibling | 1 | – | 2.8 | 5.2 |
Dearden et al. (2001) | Cy/TBI | PR 2 | Sibling | 4 | 25 | n.a. | 2+,3,16+, 24+ |
FDR/Mel/alemtuzumab | 1st CR 2 | ||||||
Murase et al. (2003) | Bu/Cy | PR | MUD | 1 | – | n.a | 16+ |
Tanimoto et al. (2005) | Cy/TBI | PD | UCB | 1 | – | n.a. | 9+ |
de Lavallade et al. (2006) | RIC | PR | Auto | 1 | – | n.a. | 38 months |
F/Bu/ATG | |||||||
Kalaycio et al. (2010) | Bu/TBI various | Various | PB and BM | 21 | 28 | 5,1 | (11,2 months)a |
Krishnan et al. (2010) | Mel or Cy/TBI various | PR | Auto | 15 | 7 | 28 | 52 |
Allo | 13 | 31 | 24 | 33 | |||
Wiktor-Jedrzejczak et al. (2012)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|