Indolent subtypes
Frequency (%)
Aggressive subtypes
Frequency (%)
Mycosis fungoides variants and subtypes
61
Sézary syndrome
4
Folliculotropic mycosis fungoides
6
Adult T-cell leukemia/lymphoma
<1
Pagetoid reticulosis
<1
Extranodal NK/T-cell lymphoma, nasal type
<1
Granulomatous slack skin
<1
Primary cutaneous peripheral T-cell lymphoma, unspecified
3
Primary cutaneous CD30+ lymphoproliferative disorders
26
Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma (provisional)
<1
Primary cutaneous anaplastic large-cell lymphoma (C-ALCL)
10
Cutaneous γ/δ T-cell lymphoma (provisional)
<1
Lymphomatoid papulosis (LyP)
16
Primary cutaneous CD4+ small-/medium-sized TCL
3
Subcutaneous panniculitis-like T-cell lymphoma (provisional)
1
Improvements in classification and the development of new drugs during the recent years have also led to more differentiated disease and stage-specific treatment options that allow for effective disease control in many cases.
8.2 Mycosis Fungoides
First described in 1818 by Alibert (Alibert 1818), Mycosis fungoides (MF) represents the prototype of CTCL. It is characterized by a variety of more or less specific skin findings that can occur sequentially in a stage-dependent manner but also present synchronously in more advanced stages. It is in most cases a chronic indolent lymphoma and many patients will not progress to a nodal or systemic involvement of the disease.
The diagnosis can be difficult and particularly in the very early stages, repeated biopsies and clinical follow-up may be necessary in order to differentiate it from benign eczematous disorders or certain types of parapsoriasis. Once established, the disease can be managed with a variety of skin-directed and systemic treatment options.
8.2.1 Epidemiology
MF occurs worldwide and it is the most common form of CTCL accounting for approximately 60–75 % of CTCL cases. The incidence of CTCL is estimated to be around 0.3–0.6 per 100,000 persons per year in different countries (Criscione and Weinstock 2007; Saunes et al. 2009). Increases in incidence of CTCL up to 1.0/100,000/year have been reported (Bradford et al. 2009), but the significance of these findings is uncertain. Regarding MF, males are more often affected than females with a ratio of around 1.3–1.6, and the incidence of MF is higher among blacks as compared to white or hispanic patients.
8.2.2 Clinical Presentation
The clinical presentation of MF in many cases follows the classical presentation of Bazin (Bazin 1870) with (1) erythematous patches, (2) plaques, and (3) tumors (Fig. 8.1). While in many cases there is an evolvement from (1) to (2) to (3) over time, all three clinical presentation can be present in parallel in advanced cases.
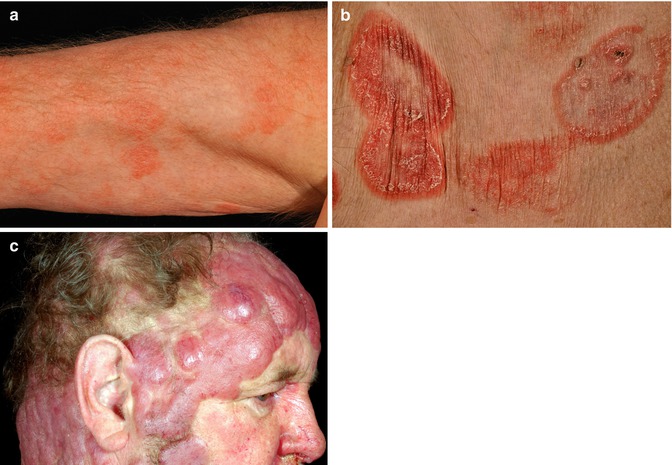

Fig. 8.1
Mycosis fungoides—clinical appearance. (a) Patches. (b) Plaques. (c) Tumor stage
In the early stage of MF, many patients report a long-standing history of nonspecific erythema without further clinical symptoms. During this phase the histological diagnosis may be hard to establish, and the term “premycotic” stage has been used in this clinical situation. As a complicating fact, the so-called parapsoriasis disorders show some relationship to MF. The term was introduced in the beginning of the last century to describe a set of skin diseases that bear some similarities to psoriasis, lichenoid dermatosis, and MF while often being chronic, symptomless, and benign. While the large plaque type of “parapsoriasis en plaque” is now regarded as an early form of MF, in many cases the controversy over the so-called small plaque parapsoriasis is ongoing. While some authors tend to call this disease “chronic superficial dermatitis,” others consider it as an early and/or abortive form of MF. While the majority will persist as a chronic benign condition, there are several cases of evolution into MF.
The diagnosis of early MF often requires an experienced clinicopathological review of the findings. Frequently, repeated biopsies will be necessary to establish a firm diagnosis.
Traditionally MF stages have been described according to their clinical appearance, including early, eczema-like “patches”; infiltrated plaques; and eventually “fungoid” (mushroom-like) tumors (Fig. 8.1). Erythroderma has been observed in patients with CTCL as part of the clinical spectrum with Sézary syndrome (SS) representing a particular type of erythrodermic CTCL accompanied by gross lymph node involvement and leukemic spread.
8.2.3 Morphology
Patch-stage MF is characterized by a superficial, dermal band-like distribution of small- to intermediate-sized lymphocytes with irregular to cerebriform nuclei, condensed chromatin, and scant cytoplasm (Fig. 8.2) (Smoller et al. 1995). Macrophages may be intermixed with the lymphocytes but granulocytes are rarely seen. Individual lymphoid cells often extend into the lower epidermis (epidermotropism). The epidermal keratinocytes show only minimal change in response to the infiltrating tumor cells. Plaque-stage MF is characterized by more extensive involvement of the epidermis by the neoplastic cells, often collected within small microabscesses (Pautrier microabscesses) (Smoller et al. 1995; Nickoloff 1988). Tumor-stage disease is characterized by a pronounced dermal proliferation of atypical lymphocytes (Diamandidou et al. 1998). Morphologic variants include folliculotropic MF, which targets hair follicles and generally spares the epidermis, and pagetoid reticulosis (Woringer-Kolopp disease) which shows a marked epidermotropism and a generally good prognosis (Haghighi et al. 2000). When >25 % of the lymphoid infiltrate consists of large-sized cells, CTCL is considered as having undergone large-cell transformation (Diamandidou et al. 1998). Lymph node involvement or extensive peripheral blood involvement is associated with higher stages of disease and are poor prognostic indicators (Willemze et al. 2005).
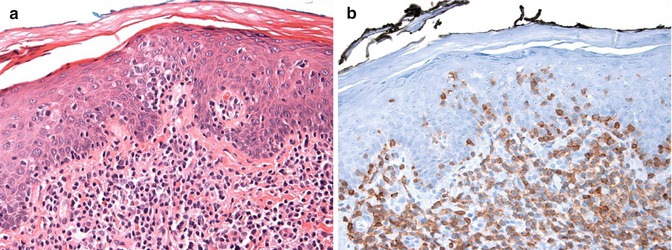

Fig. 8.2
Mycosis fungoides—histology. (a) Stained with hematoxylin and eosin stains and showing a superficial dermal and intraepidermal infiltrate of small lymphocytes and (b) stained with anti-CD3 antibody and showing a predominance of T cells
8.2.3.1 Differential Diagnosis
The sparse lymphoid infiltrate that characterizes skin lesions in early patch-stage MF can be difficult to distinguish from a normal, reactive immune response. However, a mixed cellular infiltrate consisting of plasma cells, granulocytes, and B lymphocytes in addition to T lymphocytes is more characteristic of an immune response than MF. Difficult cases can be further evaluated by molecular testing for T-cell receptor (TCR) clonality. Advanced-stage or transformed MF that is characterized by a profound infiltrate of highly atypical lymphoid cells can be difficult to distinguish from cutaneous anaplastic large-cell lymphoma (C-ALCL); lymphomatoid papulosis (LyP); cutaneous involvement by peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS); or cutaneous involvement by ALK-negative, anaplastic large-cell lymphoma (ALK-negative ALCL). Careful assessment of the patient’s medical history, tumor staging, and previous pathology is necessary to distinguish among these diagnostic possibilities.
8.2.4 Genetics
The neoplastic lymphocytes of MF, SS, C-ALCL, and up to 60 % of LyP demonstrate a clonal rearrangement of their T-cell receptor genes by PCR analysis (Ponti et al. 2005). Primary gamma-delta T-cell lymphoma (PGD-TCL) shows clonal rearrangement of the TCRδ locus (Przybylski et al. 2000).
Karyotypes of tumor cells from advanced SS often reveal complex abnormalities. Losses of TP53, p16ink4a, and PTEN genes have been associated with disease progression. However genetic lesions specific to MF and SS have yet to be described (Mao et al. 2003). In contrast, between 20 and 30 % of C-ALCL demonstrate rearrangement of the IRF4 locus by fluorescent in situ hybridization (FISH) using specific probes. This genetic lesion is only rarely seen in LyP and transformed MF, and not seen in systemic ALCL (Wada et al. 2011).
8.2.5 Staging
In 1979, the WHO/UICC and AJCC published a staging system based on what was called the Mycosis Fungoides Cooperative Group (MFCG) classification (Mycosis fungoides cooperative study 1975), which was modified by the EORTC and the International Society for Cutaneous Lymphoma (ISCL) in 2007 (Olsen et al. 2007) and eventually adapted by the TNM staging manual in 2010 (Table 8.2A) (Edge et al. 2010).
Table 8.2
Staging of mycosis fungoides and sezary syndrome as adapted by 2010 TNM maual
A: ISCL/EORTC TNM classification | ||||
Skin | ||||
T1 | Limited patches, papules, and/or plaques covering <10 % of the skin surface. | |||
May further stratify into T1a (patch only) vs. T1b (plaque ± patch) | ||||
T2 | Patches, papules, or plaques covering ≥10 % of the skin surface. May further stratify into T2a (patch only) vs. T2b (plaque ± patch) | |||
T3 | ≥1 Tumor (≥1 cm diameter) | |||
T4 | Confluence of erythema covering ≥80 % of body surface area | |||
Nodes | ||||
N0 | No clinically abnormal peripheral lymph nodes; biopsy not required | |||
N1 | Clinically abnormal peripheral lymph nodes; pathology Dutch grade 1 or NCI LN0–2. May further stratify into N1a (clone negative) vs. N1b (clone positive) | |||
N2 | Clinically abnormal peripheral lymph nodes; pathology Dutch grade 2 or NCI LN3. | |||
May further stratify into N2a (clone negative) vs. N2b (clone positive) | ||||
N3 | Clinically abnormal peripheral lymph nodes; pathology Dutch grades 3–4 or NCI LN4; clone positive or negative | |||
Nx | Clinically abnormal peripheral lymph nodes; no histologic confirmation | |||
Visceral involvement | ||||
M0 | No visceral organ involvement | |||
M1 | Visceral involvement (pathology confirmation and organ involved should be specified) | |||
Blood involvement | ||||
B0 | Absence of significant blood involvement: ≤5 % of peripheral blood lymphocytes are atypical (Sézary) cells. | |||
May further stratify into B0a (clone negative) vs. B0b (clone positive) | ||||
B1 | Low blood-tumor burden: >5 % of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2 | |||
May further stratify into B1a (clone negative) vs. B1b (clone positive) | ||||
B2 | High blood-tumor burden: ≥1,000/μL Sézary cells with positive clone | |||
B: Revised nodal staging for lymph node involvement in MF/SS | ||||
Dutch system | NCI Grading | |||
N1 | Grade 1: dermatopathic lymphadenopathy (DL) | LN0: no atypical lymphocytes | ||
LN1: occasional and isolated atypical lymphocytes (not arranged in clusters) | ||||
LN2: many atypical lymphocytes or in 3-6 cell clusters | ||||
N2 | Grade 2: DL w/ early involvement by MF (presence of cerebriform nuclei > 7.5 μm) | LN3: aggregates of atypical lymphocytes; nodal architecture preserved | ||
N3 | Grade 3: partial effacement of LN architecture; many atypical cerebriform mononuclear cells (CMCs) | LN4: partial/complete effacement of nodal architecture by atypical lymphocytes or frankly neoplastic cells | ||
Grade 4: complete effacement | ||||
C: Clinical staging schema | ||||
T | N | M | B | |
IA | 1 | 0 | 0 | 0,1 |
IB | 2 | 0 | 0 | 0,1 |
IIA | 1,2 | 1,2 | 0 | 0,1 |
IIB | 3 | 0-2 | 0 | 0,1 |
IIIA | 4 | 0-2 | 0 | 0 |
IIIB | 4 | 0-2 | 0 | 1 |
IVA1 | 1-4 | 0-2 | 0 | 2 |
IVA2 | 1-4 | 3 | 0 | 0-2 |
IVB | 1-4 | 0-3 | 1 | 0-2 |
The skin (T) status includes patches and plaques grouped together and graded by the extent of body surface area (BSA) involvement (T1 < 10 %; T2 ≥ 10 % BSA). Tumor stage (T3) and erythroderma (T4) will lead to an upstaging of the disease. Remarkably, only a minority of patients will undergo a formal transition to T3 and consequently T4. Rather, the development of tumors (T3) is associated with further nodal or visceral organ involvement in a proportion of cases, while most T4 cases develop directly from T1/T2 and are associated with the risk of blood (B) involvement, sometimes called “secondary” Sézary syndrome (Quaglino et al. 2012).
For nodal involvement a revised grading system has to be applied (Table 8.2B) either using the NCI-LN (Sausville et al. 1985; Vonderheid et al. 1994b) or the so-called Dutch system (Scheffer et al. 1980). They both take into account the observation that enlarged lymph nodes in MF might represent an initially benign infiltration of what has been called the dermatopathic lymphadenopathy which can also be observed in inflammatory skin disease. Nevertheless, the presence of dermatopathic lymphadenopathy is a significant predictor for the development of further nodal progression (Quaglino et al. 2012) and therefore leads to upstaging.
8.2.6 MF Subtypes
The clinical variants of the classical clinical presentation of MF show a broad range of different clinical pictures. One of the very early descriptions of MF variants is that of disease occurring primarily as tumors thereby sparing the patch/plaque stages, formerly termed MF “d’emblée” (Vidal and Brocq 1885). Meanwhile these forms are mainly considered as representing either pleomorphic CTCL, anaplastic CD30-positive CTCL, or as CTCL, unspecified, and the term “d’emblée” is abandoned by most authors (Keehn et al. 2007; Olsen et al. 2007). During the past decades a wide range of additional clinical and/or histopathological subtypes have been described ranging from only subtle clinicopathological variations of classical MF to distinct entities that deserve particular diagnostic and therapeutic attention.
In the following sections we will describe the variants that have a significant impact on diagnosis, prognosis, or treatment.
8.2.6.1 Folliculotropic MF
Patients with folliculotropic MF (fMF) exhibit a particular pattern of neoplastic infiltration of the skin. Hair follicles are predominantly affected and the invasion of the hair follicle epithelium leads to characteristic clinical findings including acne-like cysts and comedones as well as a certain pattern of hair loss which is histologically often accompanied by mucin deposits, so-called mucinosis follicularis. While follicular mucinosis has sometimes been described as being a specific finding in fMF, there are clear examples of nonmalignant follicular mucinosis in the literature, in particular in children (Zvulunov et al. 2012). Conversely, fMF may occur without signs of mucinosis (van Doorn et al. 2002).
8.2.6.2 Pagetoid Reticulosis/Unilesional MF
Historically, the term pagetoid reticulosis was referred to as a specific infiltration pattern of this subtype of T-cell lymphoma. However, lately it has been distinguished into a localized (Woringer-Kolopp) and a disseminated (Ketron-Goodman) variant. Nowadays, the latter term is obsolete and this subtype is generally included in the classical MF, as the histological features are not as distinctive, and some cases that were reported would now be classified as CD8-positive cytotoxic CTCL. Likewise, there is no sharp distinction between pagetoid reticulosis and cases that have been published as unilesional MF, since they apparently lacked the histopathological features of pagetoid reticulosis.
Typically the unilesional variants including classical pagetoid reticulosis present as a single plaque with predilection for the lower leg as a site. This is a chronic disease with no propensity to spread systemically and has an excellent prognosis (Steffen 2005).
8.2.6.3 Granulomatous MF and Granulomatous Slack Skin
Granulomatous forms of MF have repeatedly been reported, and in 1978 a granulomatous variant with particular features was named “granulomatous slack skin” (GSS) (Ackerman 1978), sometimes also called “cutaneous elastolytic lymphoma.” Clinically the disease shows features of cutis laxa with abundant skin folds overlying the infiltrative process. It is often observed in the big flexures of the axillary or inguinal regions and the course is usually mild. However, a remarkable association with preceding, synchronous, or subsequent Hodgkin’s or other lymphoproliferative disease including classical MF has been reported (Clarijs et al. 2003).
It could be shown in a larger series of cases that despite some clinical differences, granulomatous MF and GSS show an overlapping histological spectrum. Usually there is a diffuse dermal infiltrate of small or small- to medium-sized lymphocytes with cerebriform nuclei. Epidermotropism is often absent. Sarcoid-like granuloma formation and scattered multinucleated giant cells are prominent along with loss of elastic fibers and phagocytosis of elastic fibers by histiocytic giant cells. No feature was found to discriminate between granulomatous MF and GSS based on histological findings alone (Kempf et al. 2008).
8.3 Sézary Syndrome
Sézary and Bouvrain described this CTCL variant in 1938 based on the classical triad of (1) erythroderma, (2) generalized lymphadenopathy, and (3) leukemic spread of a particular type of neoplastic cells (Sézary and Bouvrain 1938). The definition of Sézary syndrome (SS) has been changing over the years, and newer technologies and biomarkers have been introduced to differentiate SS from erythrodermic MF.
8.3.1 Epidemiology
Sézary syndrome accounts for approximately 5 % of CTCL cases and affects mainly the elderly population with a mean age at presentation of 66 years. Like in MF, there is an approximately 1.6:1 preponderance for males and unlike MF caucasians are more likely to be affected than individuals of colored skin (Kubica et al. 2012).
8.3.2 Clinical Presentation
The clinical presentation includes erythroderma with a total body surface involvement of 80 % or more (Fig. 8.3). Frequently palmoplantar hyperkeratosis, hair loss, and extensive nail changes can be found. Pruritus, virtually present in all patients, is often much more pronounced than in other forms of CTCL. Some patients present with concurrent cutaneous tumors or plaques or have a history of an MF diagnosis, though it has been a matter of debate whether these patients have “true” SS.
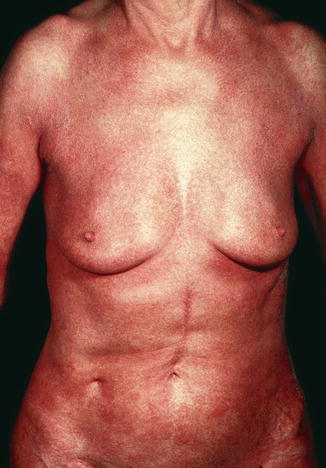

Fig. 8.3
Sézary syndrome—clinical appearance
The distinction between erythrodermic MF and Sézary syndrome has been notoriously difficult, and the discussion whether SS represents a particular variant of MF was a long-standing matter of debate. Recently it has been shown that both MF and SS seem to develop from different precursor cells (Campbell et al. 2010). In MF the phenotype of the malignant clone is compatible with an effector memory T cell baring additional skin-homing receptors which may explain the long-standing confinement of MF to the skin and eventually the skin-draining lymph nodes. In contrast, a central memory T cell-like phenotype can be found in Sézary syndrome, suggesting that both MF and SS are different entities. As a consequence, erythrodermic MF is also assigned a different disease stage than a fully developed Sézary syndrome.
8.3.3 Morphology
The neoplastic cells, like those in MF, consist of small- to intermediate-sized lymphoid cells with convoluted or “cerebriform” nuclei, condensed chromatin, inconspicuous nucleoli, and scant cytoplasm. Despite widespread skin involvement, epidermotropism may or may not be prominent. Involved lymph nodes show gross replacement of the normal architecture by tumor cells, but bone marrow may demonstrate only a sparse, interstitial infiltrate (Scheffer et al. 1986).
8.3.4 Prognosis
The prognosis of SS is much worse than for MF with a 5-year survival rate being reported between 10 and 50 %. Much of the heterogeneity regarding the incidence and prognosis is thought to be related to the diagnostic criteria differentiating SS from erythrodermic forms of MF. The recent revision to the staging of MF and SS has given consensus based diagnostic criteria especially for the grading of blood involvement. Following this definition, significant blood involvement requires at least 1,000/μl tumor cells, either by blood smear counts of “Sézary cells” or by quantifying a characteristic aberrant population via FACS analysis.
Using strict diagnostic criteria for the diagnosis of SS, the prognosis remains poor despite new treatment options with a median survival of 4 years after diagnosis (Kubica et al. 2012).
8.4 Treatment of Mycosis Fungoides and Sézary Syndrome
Early aggressive therapy is not warranted in the management of MF as it has not been shown to impact survival (Kaye et al. 1989). Hence, early-stage disease can often easily be managed for years by topical therapies that can improve symptoms and skin appearance. These treatments are most appropriate for early-stage T1 and T2 disease states and can also be combined with systemic treatments for late-stage disease.
8.4.1 Skin-Directed Treatment
8.4.1.1 Topical Steroids
Topical or intralesional steroids are a mainstay of initial therapy for many patients with early patch-/plaque-stage disease and can provide good control for several years (Zackheim et al. 1998). Topical application to the sites of disease ensures minimal systemic absorption and side effects. Highly potent group I (US system) or class IV (non-US) steroids are the best option and it is recommended that they be applied vigorously to the lesions twice a day. Occasionally it is beneficial to use occlusive therapy especially at night in addition to the topical therapy. The treatment should be continued for at least 2–3 months to assess maximal response. At least one prospective study has looked at the response rates of topical steroids in MF patients and has reported complete response rates of 60–65 % in T1 disease and a partial response of 30 % and a CR rate of 25 % and PR of 57 % in T2 disease (Zackheim et al. 1998). Intralesional steroids can be used in the treatment of thicker lesion or tumor deposits.
8.4.1.2 Topical Cytotoxic Agents
This includes topical mechlorethamine (nitrogen mustard) and topical carmustine. The former is used more often usually in an aqueous or ointment preparation that can be cumbersome to prepare and apply. Care must be taken to avoid contact with family members and other house hold contacts. Long-term remissions lasting 4–14 years have been documented with aggressive topical therapy including a maintenance schedule for stage 1A and 1B (Vonderheid et al. 1989) but carries the risk of skin irritation and secondary malignancies. Topical nitrogen mustard has been used in sequentially or in combination therapy with TSEB (Price et al. 1977), systemic chemotherapy, and following other treatments as maintenance.
8.4.1.3 Topical Bexarotene
This is a useful therapy for patients with a limited number of patch-/plaque-type lesions (Breneman et al. 2002). The recommended dose is a 1 % gel applied twice a day to the affected areas. There are no systemic side effects and any adverse events are mild and limited to the site of application. These include skin irritation and generally increase with gel exposure. Overall response rates are around 63 % with a CR of 21 % with a median time to respond of 20.1 weeks (range 4.0–86).
8.4.1.4 Other Topical Agents
These include agents like imiquimod, an immune response modifier that is a potent inducer of interferon alpha at the site of administration. A response rate of 50 % has been demonstrated with the topical use of this agent in early-stage MF (Deeths et al. 2005).
8.4.2 Phototherapy
PUVA and UVB are the two most common forms of phototherapy used in the treatment of MF usually for widespread disease that has failed to respond or is too extensive for topical therapy. As the main mechanism of action, induction of apoptosis of exposed cells is assumed in both modalities (Weichenthal and Schwarz 2005).
In PUVA, ingested psoralen is activated by exposure to UV light at a wavelength range of 320–400 nm resulting in its binding covalently to DNA forming bifunctional adducts to pyrimidine bases. This results in lymphocyte toxicity and a decrease in the number of helper T cells. Response rates are over 95 % with CR rates of 58–83 % (Berthelot et al. 2008). A taper and maintenance schedule is recommended after the initial therapy and responses can last for a median duration of 43 months. Side effects include nausea, photosensitivity accelerated photodamage to skin, and an increased risk of melanomas and squamous cell malignancies of the skin (Lindelöf et al. 1999).
Narrowband UVB (NBUVB) at 311 nm does not require the use of a sensitizing agent and suppresses Langerhans cells and cytokine production and has largely replaced the use of broadband UVB (290–320 nm). NBUVB is more readily available and avoids the side effects of psoralen, i.e., nausea and sun photosensitivity. It is less effective in thicker lesions as compared to PUVA. Phototherapy can be combined with other therapies, including interferon and retinoids, and has a role as maintenance therapy after the use of other modalities like chemotherapy (Rupoli et al. 1999; Stadler et al. 1998; Quiros et al. 1997).
8.4.3 Radiation Therapy
CTCL are radiosensitive tumors, and for most patch-/plaque-stage disease, the target volume of treatment, i.e., epidermis and dermis, can be only a few mm in depth, meaning that most lesions can be treated with low-penetrance beams like 50–145 kvp or 4–9 MeV electron beams. Deeper lesions like tumors and ulcers require higher energy beams. The dose of radiation is determined by the goals of treatment. Effective palliation of lesions can be achieved by 15–20 Gy though there is a dose response effect and higher doses are required to completely clear the lesions. Durable remissions after RT alone are rare except in cases of T1 lesions in a bathing trunk distribution that can be “cured” with long-term remission with RT doses of up to 30cGY. The 5-year relapse-free survival after RT is 40–60 % for T1 disease but less than 10 % for T4 disease. XRT is an excellent option for palliation and pain control of large tumors and ulcerated lesions. Radiation can be given concurrently with many other agents including retinoids, antibodies, and several chemotherapy agents though the dose of these agents may need to be modified.
8.4.3.1 Total Skin Electron Beam (TSEB) Therapy
This is an effective palliative strategy for patients with extensive skin and blood involvement. Best results are seen at doses of 2,500–3,000 Gy given on a fractionated regimen of 32–36 Gy with appropriate shielding over a time period of 9 weeks given at centers that are experienced in the technique. Side effects include skin erythema, hair loss, and nail dystrophy. Some patients will also have decreased sweating and changes in body temperature control that may be long lasting. Combination of TSEB with chemotherapy has been studied with the best results seen in patients receiving chemotherapy followed by TSEB.
8.4.4 Systemic Treatment
Once skin-directed therapies fail or if the disease is advanced (IIB and beyond), it becomes necessary to start systemic therapies. The principles of treatment are to minimize immunosuppression, reduce the risk of infections, and palliate symptoms. The disease remains largely incurable unless the patient undergoes an allogeneic stem cell transplant; hence it is prudent to select therapies that can be given for prolonged periods of time and have minimal side effects. Many of the treatments can be used in a recurrent setting and combination therapies are encouraged in progressive disease. Consensus-based guidelines are used to determine treatment options in a given situation.
8.4.4.1 Biologic Agents
Interferon
Interferons are a class of TH1 cytokines and function as immune modifiers. Recombinant interferons are a good therapeutic option for patients with mycosis fungoides for all stages of disease including SS. Alpha interferon is the most common formulation available for clinical use and can be given subcutaneously or intramuscularly and has also been used for intralesional injections. The usual dose is three million to ten million units given subcutaneously in various schedules that range from three times a week to daily dosing. Data from studies involving more than 12 patients have reported partial response rates of 17–53 % and complete remission rates of 4–27 %. These studies included varying stages of disease (Olsen 2003), and the responses were higher if this was the first line of systemic therapy (Bunn and Norris 1990). It has limited and reversible dose-dependent side effects that include fever, chills, influenza-like symptoms, myalgias, and arthralgias. More chronic effects include fatigue, anorexia, weight loss, sleep disturbance, and hepatitis. Alpha Interferon can be used alone or can be combined with other treatment modalities to improve response rates and outcomes. These include extracorporeal photopheresis (Olsen et al. 1989), low-dose chemotherapy (Foss et al. 1992, 1994), retinoids (Stadler et al. 1998; Knobler et al. 1991), and phototherapy (Rupoli et al. 1999). While alpha interferon is the most commonly used formulation, there is data using interferon gamma as well in the treatment of MF resulting in prolonged responses (Kaplan et al. 1990).
Interleukins
Interleukins as immune response modifiers that can be used in the treatment of MF. Interleukin-2 and interleukin-12 have been used in clinical trials with good responses, but the excessive toxicity and limited availability make them impractical for general use (Duvic et al. 2006c).
8.4.4.2 Thalidomide-Derived Immunomodulatory Drugs (IMiDs)
Lenalidomide is currently being used in various hematological malignancies and solid tumors. The mechanism of action is unknown but appears to be immune-mediated with stimulation of T- and NK cell function, induction of Th1 cytokine production, and cytotoxic activity. A phase II trial in relapsed CTCL showed a RR of 32 % with partial remissions and stabilization of disease for a median of 5 months. A decrease was noted in the number of CD4+ T cells and CD4+ CD25+ T-regulatory cells and seemed to correlate with response (Querfeld et al. 2011). The main side effects are myelosuppression and an increased incidence of thrombotic events.
8.4.4.3 Proteasome Inhibitors
Proteasome inhibitors are a new group of anticancer agents that block the proteasome degradation system resulting in effects on cell survival pathways and apoptosis. The following 2 proteasome inhibitors are in clinical use for MF/SS.
Bortezomib
Bortezomib is a dipeptide boronic acid that binds the catalytic site of the 26S proteasome with high affinity and specificity (Bonvini et al. 2007). This agent is used in the treatment of MF/SS but the data supporting its use is limited to one study of ten patients. Bortezomib was administered at a dose of 1.3 mg/m2 IV on days 1,4,8, and 11 every 21 days for 6 cycles, and an ORR of 70 % was noted with 1 CR lasting over 12 months (Zinzani et al. 2007). The main side effects associated with its use are myelosuppression, particularly thrombocytopenia, and sensory neuropathy that was seen in 50 % of the patients treated on the MF study. Other effects include diarrhea, asthenia, and headaches. A subcutaneous route of administration is being explored in other diseases and has shown to be just as efficacious but compared to IV infusions is associated with a lower incidence of neuropathy (38 % vs. 53 % P = 0.044) (Moreau et al. 2011). Similar trials are warranted in MF.
Carfilzomib
Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S proteasome, an enzyme that degrades unwanted cellular proteins. It is very well tolerated and can be administered for prolonged time periods without significant effects of neuropathy or myelosuppression. Current trials are under way to establish its activity in this group of lymphomas in combination with other agents (ClinicalTrials.gov Identifier: NCT01276717, (Dasmahapatra et al. 2011).
8.4.4.4 Retinoids
Retinoids are derivatives of vitamin A that bind to retinoid receptors in the nucleus and trigger downstream events of transcription, cell differentiation, and apoptosis (Mukherjee et al. 1997). Retinoid receptors come in two major flavors, i.e., retinoic acid receptor (RAR) and the retinoid X receptor (RXR) with isotypes (a,b,gamma) that vary in the degree of expression in different tissues. Skin tissues express both RAR and RXR receptors and various retinoids are in use for various skin disorders including MF and SS.
Bexarotene
This is a synthetic retinoid that selectively binds to the RXR receptors and is formulated both as an ointment for topical use as well as an oral formulation. Both forms are approved for the treatment of MF/SS both in the USA and Europe for both early-stage disease and advanced disease including SS (Talpur et al. 2002; Breneman et al. 2002; Duvic et al. 2001b; Gniadecki et al. 2007). Response rates and side effect profiles are dose dependent. In phase II/III studies response rates of 54 % were observed at a dose of 300 mg/m2 per day and up to 67 % at higher doses (Duvic et al. 2001b) in early-stage MF (stage I–IIA). For advanced-stage disease (IIIB–IVB) the response rate was 48–55 % (Duvic et al. 2001b). The median time to respond was noted to be 8.1 weeks (range 4–16) and 25.7 weeks (2–28), respectively. Main side effects are reversible hyperlipidemia and hypercholesterolemia occurring within 2–4 weeks of initiating therapy that often require therapy with lipid-lowering agents, a decrease in thyroid-stimulating hormone (TSH) resulting in reduced levels of T4, hepatitis, anemia, leucopenia, headache, and dry skin. Bexarotene is contraindicated in pregnancy due to its effect on fetal development (Duvic et al. 2001a, b).
Other Retinoids
These are non-RXR selective and include oral etretinate, arotinoid, acitretin, and isotretinoin (13-cis-retinoic acid). There are no comparative trials but overall response rates based on studies range from 5 to 65 % either as single agents or in combination with PUVA, interferon, or cytotoxic chemotherapy (Burg and Dummer 2000; Zachariae et al. 1982; Stadler et al. 1998; Thomsen et al. 1989; Knobler et al. 1991).
8.4.4.5 Antibodies
Alemtuzumab (Anti-CD52)
Alemtuzumab is a humanized monoclonal antibody targeting CD52 on the surface of lymphocytes that has activity against many T-cell lymphoproliferative disorders (Piccaluga et al. 2007; Rowan et al. 1998). Alemtuzumab is thought to mediate its effects through antibody-dependent cellular toxicity and activation of complement-dependent and complement-independent cytolysis (Rowan et al. 1998; Dyer et al. 1989). Initial trials in heavily pretreated patients with MF and SS have reported response rates of 55 % with 31 % CRs (Lundin et al. 2003) and a duration of response of less than 12 months. Another small trial of eight patients reported a response rate of only 31 % with a median duration or response lasting 4 months (Kennedy et al. 2003). Main toxicity is hematological and an increase incidence in infections including CMV and EBV reactivation. Alternative dosing and routing schedules have been attempted including subcutaneous administration to reduce the associated toxicity (Bernengo et al. 2007; Zinzani et al. 2005; Querfeld et al. 2009). Alemtuzumab has shown particularly high-response rates of 86–87 % with a CR seen in 37 and 21 % cases, in small studies focused on patients with erythroderma and SS (Lundin et al. 2003; Bernengo et al. 2007; Querfeld et al. 2009), indicating that this may be an effective therapy in otherwise difficult to treat patients with SS.
Zanolimumab (Anti-CD4)
Zanolimumab (HuMax-CD4) is a humanized monoclonal antibody directed against CD4 expressed universally on helper T cells and blocks the interaction of CD4 receptor and the major histocompatibility complex class II on cells thus preventing the activation of the T cell. It results in cell death via antibody-dependent cellular toxicity (ADCC) but does not induce complement-dependent cytotoxicity (CDC). It results in depletion for CD4-expressing T cells and has been studied in the setting of MF and SS. Two simultaneous phase 2 multicenter trials were conducted in patients with CTCL, one for early-stage disease and the other for advanced disease. Responses were seen in patients with MF and SS, with a median response rate of 56 % and a median duration of response at 81 weeks with more responses noted at the higher dose level. The agent was well tolerated with mild eczema and low-grade infections in spite of effective lowering of the CD4 count in patients (Kim et al. 2007). Further evaluation of this promising agent is warranted.
Anti-CCR4
CCR4 is a chemokine receptor expressed on CD4+ helper T cells and regulatory cells (Tregs) and in varying proportions in T-cell malignancies (Imai and Umezu 1999; Iellem et al. 2001; Ito et al. 2009). KW-0761 is a humanized monoclonal antibody directed against CCR4 that has a defucosylated Fc region that enhances the ADCC due to increased binding affinity to the Fc gamma receptor on cells. A phase 1 study of the antibody given once a week for 4 weeks indicated promising responses in CTCL (Yamamoto et al. 2010).
8.4.4.6 Conjugated Antibodies
Denileukin Diftitox
Denileukin diftitox (Ontak) is a novel fusion protein consisting of the membrane translocation sequence for the diphtheria toxin and the receptor-binding sequence of the human interleukin-2 that has affinity for the human IL-2 receptor (Williams et al. 1990; Taniguchi and Minami 1993). Initial phase I/II trial confirmed the antitumor activity of denileukin diftitox in patients with CTCL (Saleh et al. 1998). A phase III trial comparing two dose levels of denileukin diftitox at 9 and 18 μg/kg given daily for 5 days every 21 days was conducted in patients with CD25-expressing CTCL and SS. This trial led to the accelerated approval of the agent by the FDA in the USA for the treatment of relapsed and refractory CTCL with more than 25 % expression of CD25. A response rate of 30 % (20 % PRs and 10 % CRs) was reported in the trial. The median duration of response was 6.9 months (2.7–46.1 months) with no difference between the two dose levels. The main side effects were flu-like symptoms, infusional sensitivity reactions, vascular leak syndrome, hypoalbuminemia, and transaminitis with a statistical hint that the side effects may be worse in the higher-dose arm (Olsen et al. 2001). A second placebo-controlled phase III trial was conducted to evaluate the efficacy of the two dose levels of denileukin diftitox, i.e., 9 and 18 μg/kg, compared with placebo in CD25-expressing CTCL and SS patients who had received up to three prior systemic therapies (Prince et al. 2010). One hundred and forty-four patients were enrolled. The ORR was 44 % (34 % PRs, 10 % CRs) with the response rate being higher in the 18μg/kg group, i.e., 49 % vs. 37.8 % in the 9μg/kg group vs. 12 % in the placebo group. Progression-free survival was 124 days better in both dose groups as compared to placebo. There was no difference in the side effect profile at the two doses. This led to the full approval of the agent in 2010 for the treatment of CTCL if there is expression of CD25. The recommended dose of the agent is either 9 or 18μg/kg and is left to the discretion of the treating physician. There is a black box warning in the label for fatal vascular leak syndromes and loss of visual acuity and color vision which may not be reversible. Combination therapies have been evaluated, the most notable being the combination of denileukin diftitox with bexarotene (Foss et al. 2005). The combination was well tolerated with an overall response rate of 67 %. The study also demonstrated that even low doses of bexarotene at 150 mg/day were capable of inducing upregulation of CD25 expression which may have led to the higher response rate.
Brentuximab Vedotin
Brentuximab vedotin is an antibody conjugate consisting of a chimeric monoclonal antibody that targets CD30 (a member of the transmembrane tumor necrosis factor family of proteins) linked to the antimitotic agent monomethyl auristatin E (MMAE). The binding of the agent to CD30 results in internalization of the compound which is then released intracellularly—mitosis is interrupted and the cell undergoes apoptosis. The agent is approved at a dose of 1.8 mg/kg given once every 3 weeks for CD30+ anaplastic large-cell lymphoma including the cutaneous variant of the disease. The response rate using this agent in the relapsed setting is 87 % in ALCL. Side effects are tolerable with the most common being sensory and motor neuropathy. Variable CD30 expression is seen in MF and up to 41 % of the time in the transformed MF (Arulogun et al. 2008). Hence, it is likely that there will be efficacy of this agent in CD30-expressing MF and SS. Early trials are encouraging and a phase III randomized trial is being conducted in CD30-expressing MF patients who need systemic therapy that will compare brentuximab vedotin with standard-dose methotrexate or bexarotene (physician’s choice) in the comparator arm.
8.4.4.7 HDAC Inhibitors
Targeting histone acetylation processes has shown to be an important therapeutic intervention for the treatment of T-cell lymphomas and CTCL in particular (Bhalla 2005; Zain et al. 2010). While the exact mechanism of action is still unknown, most of these agents have shown remarkable antitumor activity in these diseases as well as clinical benefits like the effect on pruritus. While many HDAC inhibitors are in clinical trials, two are already approved by the FDA in the USA for the treatment of CTCL in the relapsed setting. A brief description of these follows below.
Vorinostat
Belongs to the class of hydroxamic acids and has both oral and IV formulations that inhibits both class I and II histone deacetylases. The recommended dosing schedule for CTCL is 400 mg orally once a day with dose adjustments recommended for toxicities. In the pivotal phase 2 trial that led to the approval of this agent, the overall response rate was 24 % with a 58 % reduction in pruritus (Olsen et al. 2001; Duvic et al. 2007). Responses were seen across all stages of diseases for stage IIB or higher. The most common toxicities are gastrointestinal, constitutional symptoms, dysgeusia, and hematological especially reversible thrombocytopenia (Mann et al. 2007). Long-term therapy with vorinostat in patients with stable disease or partial responses is feasible with manageable toxicity (Duvic et al. 2009). Combination studies using vorinostat have been conducted with promising results including a phase 1 trial of the combination of bexarotene and vorinostat (Dummer et al. 2008). Case reports of patients receiving vorinostat in addition to their ongoing therapies to improve responses have included combinations with IFN-α, phototherapy, and photopheresis (Geskin 2010).
Romidepsin
Romidepsin (FR901228, FK228, depsipeptide) is a potent HDAC inhibitor belonging to the class of cyclic peptides that mainly inhibits HDAC1 and HDAC2 class I enzymes has an intravenous formulation and is approved in the USA for the treatment of relapsed and refractory CTCL after failing at least one prior systemic therapy. The prescribing dose is 14 mg/m2 given over a 4-h infusion once a week for 3 weeks followed by a 1-week rest. Two independent phase II trials (Piekarz et al. 2009; Coiffier et al. 2012) have been conducted, and the pooled data from both these trials has shown an ORR of 34 % with a median duration of response of 15 months (Demierre 2009). One of the striking features of romidepsin is the long duration of response that extended beyond 3 years, observed in some patients even after discontinuation of the drug. Main side effects were nausea, asthenia, anorexia, vomiting, and fatigue. The drug needs to be administered with caution in patients with significant preexisting cardiac abnormalities and concomitant medications that prolong QT interval or inhibit CYP3A4. A topical formulation of romidepsin is currently in clinical trials in limited stage CTCL.
Other HDAC Inhibitors
8.4.4.8 Single-Agent Chemotherapy
Several chemotherapeutic agents have shown activity in CTCL/MF. Initial response rates with either single-agent or combination chemotherapy remain high but the responses are short lived. Given the immunosuppressive state and propensity to infections due to a compromised skin barrier, the best strategy remains to avoid multiagent chemotherapy for as long as possible and to treat with lowest possible doses of single agents to allow more frequent administration of drug and avoid systemic infectious complications. Some of the agents with the best known activity in CTCL are as follows.
Antifolates
Methotrexate
MTX has shown significant clinical activity in many types of non-Hodgkin’s lymphoma including T-cell lymphoma and has immunosuppressive properties (Olsen 1991). Low-dose methotrexate given weekly has long been used for the treatment of MF and SS. The dose is less than 100 mg a week and is usually administered orally though it can be given intramuscularly or intravenously as well.
In spite of frequent use at doses that range between 2.5 and 25 mg a week, there are few studies that have looked at the response rates. Zackheim et al. published the first report at doses of 2.5–10 mg a week and reported responses at 58 % in erythrodermic MF and 33 % in plaque-stage disease (Zackheim et al. 1996, 2003). High-dose MTX with leucovorin rescue at doses between 60 and 240 mg/m2 has shown responses up to 80 % in patients with more advanced-stage MF (McDonald and Bertino 1978). Case reports have confirmed activity of single-agent MTX in SS patients (Zackheim and Epstein 1989).
Combinations of MTX have also shown promising results though all objective data consists of small studies and case reports. Most patients treated have advanced (at least stage IIB) disease. The few published reports of these combinations have been either with biologic agents like IFN-alpha (Aviles et al. 2007) or other chemotherapy agents like etoposide (Hirayama et al. 2000) or fluorouracil (Schappell et al. 1995). No specific recommendations can be made with these small studies with very heterogeneous groups of patients. In transformed disease, there is a trend to use systematic and combination therapy including MTX-containing regimens used for aggressive lymphomas. A topical formulation is currently being investigated and has shown to be safe in an early phase 1/2 trial (Demierre et al. 2003). Major side effects are nausea, mucositis, bone marrow suppression, alopecia, hepatitis, and cirrhosis with cumulative dosing. The side effects are dose dependent and folate supplementation can help alleviate the severity of mucositis. High-dose methotrexate is combined with folinic acid (leucovorin) rescue to minimize mucositis (Olsen 1991).
Pralatrexate
Pralatrexate is a rationally designed antifolate with a higher affinity for the reduced folate carrier (RFC) that carries the molecule into the cell and a higher affinity for the enzyme folylpolyglutamate synthase (FPGS) as compared to MTX. It has shown activity in T-cell lymphomas and has been studied in CTCL in a multicenter phase I/II trial that enrolled over 54 patients in multiple centers. At 15 mg/m2 weekly given during 3 out of 4 weeks, the ORR was reported at 45 % with a median duration of response that could not be assessed due to censoring in the study design. Responses were seen in both SS and MF patients. Most common side effects were mucositis and leucopenia at this dose level (Horwitz et al. 2012).
Nucleoside Analogues
Nucleoside analogues are antimetabolites that are phosphorylated and incorporated into the growing DNA strand of a dividing cell in the S phase. Some of these agents are also active in MF and SS as described below.
Gemcitabine
This agent is widely used in the treatment of NHL and CTCL. However, published data to support the use of this agent in CTCL and SS is limited to a few small studies. The agent is given weekly for 3 weeks with a 1 week break at the end of each cycle. Dosing ranges from 1,000 to 1,200 mg/m2 given intravenously. Some of the larger studies have included up to 30 patients with advanced-stage disease and have reported responses of up to 70 % with a few CRs as well (Duvic et al. 2006d; Zinzani et al. 2000; Marchi et al. 2005; Sallah et al. 2001). Main side effects are myelosuppression, fever, nausea, vomiting, interstitial pneumonitis, alopecia, and radiation sensitivity. Cases of hyperpigmentation in SS patients have been seen. Rare incidences of hemolytic uremic syndrome have been reported.
Fludarabine
Use of single-agent fludarabine has been studied in CTCL and SS. Trials are small but response rates vary from 19 to 30 % with CR of 9 %. Response may be higher in the SS group with a RR of 35 % and CRs of 18 % (Quaglino et al. 2004). Combination chemotherapy with fludarabine has also been evaluated with interferon alpha. 35 patients were treated and 11 % (4/35) reached a CR including 11 patients with SS maintained for 18 months in 3 patients (Foss et al. 1994). Infectious complications remain the major side effect.
Cladarabine
Small case series of up to eight patients reported responses in patients with MF including 1 CR (O’Brien et al. 1994; Trautinger et al. 1999). Differing dosing schedules have been employed. The largest series consists of 24 patients with MF/SS treated at 0.1 mg/kg/day by continuous infusion over 5–7 days repeated every 28 days. The RR was 24 % with 3 out of 24 patients reaching a CR (Kong et al. 1997). Based on an analysis of several small series, a RR of 50 % has been reported in SS (Saven et al. 1992; Zaucha et al. 1997; Bouwhuis et al. 2002). Main side effects are immunosuppression and prolonged leucopenia. Occasional constitutional symptoms of fever and nausea can occur.
Pentostatin (Deoxycoformycin)
One of the most widely studied agents in CTCL as activity was established in the first phase I study of this agent (Grever et al. 1983). Several trials have been published that support the use of single-agent pentostatin in the treatment of MF and SS including ECOG and EORTC but there is no consensus on the dose or schedule. These trials are also marked by patient heterogenicity and lack of uniform response criteria. Reported response rates are 31–66 % with higher responses seen in SS (71 %) with reports of CR lasting up to 76 months (Tsimberidou et al. 2004; Cummings et al. 1991; Greiner et al. 1997; Ho et al. 1999; Kurzrock et al. 1999). A combination of pentostatin and interferon produced a response rate of 41 % in a group of 41 patients with 2 CRs in SS patients and 15 PRs (Foss et al. 1992). The most common side effects are myelosuppression, nausea, fever, and elevation of liver enzymes that is transient. Prolonged suppression of CD4 counts can occur putting the patient at risk of potentially life-threatening infections. Neurologic symptoms, pulmonary toxicity, or unexpected nephrotoxicity has been reported.
Forodesine
Two phase I/II studies of forodesine have established its activity in patients with MF/SS using an IV and an oral formulation. Small studies have reported a ORR of up to (Duvic 2007; Duvic et al. 2004, 2006a). Side effects are fatigue, edema, nausea, pruritus, dyspnea, and headaches. Lymphopenia and low CD4 counts have been noticed in patients but opportunistic infections are not common (Duvic et al. 2006b).
Alkylating Agents
Most of the alkylating agents that are used in MF/SS are part of the combination chemotherapy regimens, but single agents used at lower doses have been attempted to enable prolonged use with fewer side effects.
Single-agent use for MF/Ss has been established for the following agents:
Nitrogen Mustard (Mechlorethamine)
The first alkylating agent was used as early as 1950 to treat 21 cases of MF at 0.1 mg/kg/day for 10 days resulting in responses to initial cycles of therapy (Karnofsky 1950). Topical formulation is used extensively in early-stage disease. A 27-patient study was conducted with MF/SS patients using nitrogen mustard at varying doses for a total of 0.4 mg/kg per session along with topical nitrogen mustard. An ORR of 54 % was demonstrated with one response lasting for more than 1 year. IV nitrogen mustard can cause phlebitis, myelosuppression, rashes, and GIT disturbance (Van Scott et al. 1975).
Chlorambucil
The earliest documented use of chlorambucil in MF was in the 1960s when it was used in four patients to treat erythroderma at doses of 4.5–56 mg/kg for a 4-week cycle with clinical responses lasting 4–24 months. Several case reports of single-agent chlorambucil in MF have shown variable results at varying doses. Best results in MF/SS have been shown with concomitant use of steroids particularly in SS (Hamminga et al. 1979; Winkelmann et al. 1984) and in combination with leukapheresis (McEvoy et al. 1989) resulting in an ORR of 100 % and a DOR of 1–3 years with improved survival of 6.5–8 years as compared to historical control survival of 3 years. Pulse chlorambucil given as 10–12 mg/day with a steroid on 3 successive days every 2 weeks also produced 54 % CRs and 46 % PRS (100 % RR) in a series of 13 SS patients (Coors and von den Driesch 2000). Medians response duration was 16.5 months.
Cyclophosphamide
This was first used to treat MF/SS as early as 1960s (Abele and Dobson 1960). The initial four patients were treated at 200 mg/m2 for 14–220 days, and 3 out of 4 had a response to the initial therapy that required weekly maintenance dosing of 400–700 mg. There are several case reports in the literature regarding responses in MF/SS patients obtained with lower doses of Cytoxan used as a single agent given weekly (Suter 1964; Auerbach 1970; Maguire 1968), but the main use of Cytoxan remains as part of combination chemotherapy. The main side effects are alopecia, nausea, vomiting, and myelosuppression at higher doses. Like all alkylators, it carries the potential for germ cell damage in younger patients.
Temozolomide
Temozolomide is an oral alkylating agent that functions as a prodrug and undergoes rapid nonenzymatic conversion to active 5-(3-metyltriazen-1-yl) imidazole-4-carboxamide (Newlands et al. 1992, 1997). In a phase 1 study of this agent in advanced cancers, one patient with MF had a CR of 7 months duration (Newlands et al. 1992). This led to a prospective phase II study of nine patients at a dose of 150 mg/m2/day × 5 days for the first 28 days cycle and then 200 mg/m2 × 5 days for cycles 2 and 3. The ORR was 33 % including 1 CR and 2 PRs with a duration of response of 6–9 months (Tani et al. 2005). Myelosuppression is the main side effect with counts nadiring at day 22 of the treatment cycle (Newlands et al. 1997).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree