Fig. 14.1
Path slides to be chosen by pathologist
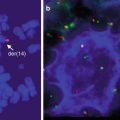
Other cytogenetic abnormalities that have been reported in MALT lymphomas include t(11;18), t(14;18), t(1;14), t(3;14), and trisomy 8. The translocation t(11;18) is the most common, occurring in 18–53 % of MALT lymphomas of any tissue and associated with low-grade histology (Auer et al. 1997; Ott et al. 1997). It results in the fusion of the apoptosis inhibitor 2 (API2) gene with the MALT1 gene whose product increases nuclear factor-κB (NF-κB) transcriptional activation of a number of genes, including ones that promote proliferation and inhibit apoptosis (Dierlamm et al. 1999; Stoffel et al. 2004). The translocation is most common in gastric MALT lymphomas, but translocation t(14;18), which pairs the MALT1 gene with the immunoglobulin heavy chain gene, has been described with increased frequency in MALT lymphomas of non-gastric sites (Dierlamm et al. 1999; Streubel et al. 2003). Translocation (1;14), on the other hand, is rarer overall but more frequent in gastric and pulmonary MALT lymphomas; it results in the overexpression of bcl-10 which also activates NF-κB and results in the transcription of genes that promote proliferation and inhibit apoptosis (Lucas et al. 2001; Willis et al. 1999). In the stomach, H. pylori-negative MALT lymphomas are enriched for the t(11;18) translocation; in addition H. pylori-negative gastric MALT lymphomas exhibit greater CpG island methylation which is presumed to result in the silencing of tumor suppressor genes and increased nuclear expression of bcl-10 and NF-κB (Kaneko et al. 2003; Liu et al. 2001; Ye et al. 2003; Yeh et al. 2005). The identification of a t(3;14) translocation in a MALT lymphoma of the thyroid prompted further analysis of additional thyroid, ocular adnexal, and cutaneous MALT lymphomas that had been known not to harbor a t(11;18), t(1;14), or t(14;18) translocation; the t(3;14) translocation was present in this cohort at an overall frequency of 10 % (Streubel et al. 2005). This translocation involves the IGH and FOXP1 genes and is thought to activate NF-κB as well. This translocation was not found in additional series of over 200 patients, but strong nuclear FOX-P1 expression has been seen regardless of whether this translocation is present in approximately 30 % of patients with marginal zone lymphoma (Goatly et al. 2008; Haralambieva et al. 2006; Remstein et al. 2006). Single-nucleotide polymorphism (SNP) arrays of MALT, nodal marginal zone, and splenic marginal zone lymphomas revealed that MALT lymphomas are more often associated with gains at chromosomes 3p, 6p, and 18p and del(6q23) than the other subtypes of marginal zone lymphoma; del(7q31) and del(8p) were more frequent in splenic marginal zone lymphoma (Rinaldi et al. 2011). All marginal zone lymphoma subtypes were associated with gains in chromosomes 3q and 18q.
H. pylori-associated gastric MALT lymphomas illustrate the process of MALT lymphomagenesis. In the stomach, H. pylori infection results in the development of gastric mucosal lymphoid tissue resulting in chronic gastritis. Although only a small percentage of these patients will go on to develop MALT lymphoma, understanding who these patients are is under investigation. There is evidence that polymorphisms in certain genes involved in inflammation and immune reactions are associated with the risk of developing MALT lymphoma following H. pylori infection, including polymorphisms in the toll-like receptor 4 (TLR4) gene, as well as genes that play a role in antioxidant capacity including IL1RN and GSTTI (Hellmig et al. 2005; Rollinson et al. 2003). MALT lymphomas of the stomach are likely antigen driven in their early phases, and the dependency on antigenic stimulation may explain why these lymphomas are unlikely to disseminate to antigen-negative tissues for long periods of time. The malignant lymphocytes have undergone somatic hypermutation suggesting that they were selected for during a secondary immune response (Du et al. 1996; Qin et al. 1995). Furthermore, H. pylori strain-specific activated T cells stimulate proliferation of gastric MALT lymphoma cells in vitro (Hussell et al. 1996). Interestingly, however, these clonal B cells do not produce antibodies that recognize H. pylori epitopes, suggesting that the antigen specificity of the immune reaction lies in the T-cell response leading to the ultimate clonal expansion of a B-cell population (Bende et al. 2005; Hussell et al. 1993). A substantial proportion of malignant B cells in MALT lymphomas express B-cell receptors with strong homology to rheumatoid factors, and this appears to be mutually exclusive with the presence of the t(11;18) translocation (Bende et al. 2005; Sakuma et al. 2007). This suggests that t(11;18)-negative MALT lymphomas are driven by stimulation of high-affinity B-cell receptors by antibody-antigen immune complexes and activated T cells, while t(11;18)-positive MALT lymphomas are not dependent on B-cell receptor signaling but instead are driven by constitutive activation of NF-κB. Interestingly and consistent with this model, t(11;18)-positive MALT lymphomas are enriched in patients without a history of autoimmune diseases (Wohrer et al. 2007).
14.3 Risk Factors and Disease Associations
Prolonged lymphoid proliferation, as is the case in settings of chronic inflammation and infection like H. pylori infections described above, is thought to result in the formation of a malignant B-cell clone that can develop into a MALT lymphoma. Inflammation is believed to result in the genesis of ectopic, organized lymphoid tissue in affected tissues as a result of the elaboration of certain cytokines and chemokines that recruit and B and T cells and facilitate the formation of germinal centers, germinal center reactions, and somatic hypermutation within activated B cells (Bende et al. 2009).
14.3.1 Chronic Inflammation
The most well-described conditions of chronic inflammation that have been associated with a risk of developing MALT lymphoma include systemic and organ-specific autoimmune diseases. In some series, up to 40 % of patients with MALT lymphoma have a history of autoimmune disease (Wohrer et al. 2007). Of these, Sjögren’s syndrome and Hashimoto’s thyroiditis have the strongest correlation with the development of MALT lymphomas of the salivary and thyroid glands, respectively (Ansell et al. 1999; Diss et al. 1995; Kassan et al. 1978; Pertovaara et al. 2001). Patients with Sjögren’s syndrome, a condition associated with inflammation of the lacrimal and salivary glands leading to dry eyes and dry mouth, are 44 times more likely to develop non-Hodgkin lymphoma than the general population, and this risk appears to be increased further in patients with elevated β2-microglobulin levels and evidence of reactive lymphadenopathy and/or splenomegaly (Kassan et al. 1978; Pertovaara et al. 2001). Biopsies of salivary and lacrimal glands from patients with Sjögren’s syndrome are notable for CD4 and CD8 T cell and CD20 B-cell infiltrates with increased expression of T- and B-cell chemokines CXCL12, CXCL13, and CCL21; the degree of elevation of these chemokines directly correlates with the extent of lymphocytic infiltration and organization (Barone et al. 2005; Salomonsson et al. 2003). The result is a local inflammatory environment marked by high interferon-γ, interleukin-2, interleukin-10, and B-cell activating factor levels, all of which promote the transcription of genes involved in cell survival and proliferation and the ultimate development of a malignant B-cell clone (Fox et al. 1994; Groom et al. 2002). A similarly organized and prolific lymphocyte infiltrate with elaboration of a T-helper-1 cytokine profile can be seen in biopsy specimens from patients with Hashimoto’s thyroiditis (Armengol et al. 2001; Del Prete et al. 1989). As a result, patients with Hashimoto’s thyroiditis are 67 times more likely to develop lymphoma of the thyroid than the general population, though thyroid MALT is a very rare disease (Holm et al. 1985). Clonal B-cell populations have been found in patients with autoimmune thyroid disease without evidence of lymphoma, and this did not progress to overt lymphoma in three such patients with over a decade follow-up, however (Saxena et al. 2004). Other autoimmune diseases have been shown to be associated with an increased risk of lymphoma and specifically marginal zone lymphoma, but to a lesser degree, including systemic lupus erythematosus which carries an eightfold increased risk of developing marginal zone lymphoma. Data regarding the risk of rheumatoid arthritis and lymphoma is conflicting, and a recent meta-analysis suggests that there is no significant association between the two diseases (Baecklund et al. 2006; Ekstrom Smedby et al. 2008; Smedby et al. 2006). Patients who develop MALT lymphomas in the context of autoimmune disease are more commonly female and of younger age, and the lymphomas are more often non-gastric and negative for the t(11;18) translocation or for trisomy 3; their prognosis appears to be similar to other MALT lymphoma patients (Wohrer et al. 2007).
14.3.2 Infection
As discussed previously, epidemiologic studies support a causal role of H. pylori infection in the development of MALT lymphomas, specifically of the stomach. In Northeastern Italy, where the incidence of H. pylori infections is high, there is an associated increased incidence of gastric lymphomas (Doglioni et al. 1992). Similarly, a case-controlled study documented an increased risk of gastric lymphoma in patients with H. pylori infection (Parsonnet et al. 1994). In addition, in some populations, the incidence of gastric MALT lymphoma and proportion of H. pylori-associated gastric MALT lymphoma is decreasing with increasing recognition and treatment of H. pylori infections (Luminari et al. 2010). As will be outlined in Sect. 14.5.1.1 of this chapter, the most compelling evidence for a causal association between H. pylori infection and gastric MALT lymphoma comes from the efficacy of H. pylori treatment in the treatment of these lymphomas (Bayerdorffer et al. 1995; Chen et al. 2005; Fischbach et al. 2004; Neubauer et al. 1997; Roggero et al. 1995; Stathis et al. 2009; Steinbach et al. 1999; Wotherspoon et al. 1993; Wundisch et al. 2005).
Infections with C. psittaci, B. burgdorferi, C. jejuni, and HCV have also been associated with an increased risk of MALT lymphoma in some populations. In a cohort of Italian patients with MALT lymphoma of the ocular adnexa, there was an increased frequency of C. psittaci in the tumor tissue and peripheral blood mononuclear cells than in healthy individuals (Ferreri et al. 2004). Seven of these patients were treated with doxycycline with eradication of the organism, and two of four evaluable patients had a documented tumor response. After this first report, many studies have been conducted showing high prevalence variations among different geographic regions (Chanudet et al. 2006; Mulder et al. 2006; Rosado et al. 2006). There are similar reports of an association between B. burgdorferi infection and cutaneous MALT lymphoma, C. jejuni infection and small bowel MALT lymphoma, and HCV infection and MALT lymphoma (Ascoli et al. 1998; Cerroni et al. 1997b; Lecuit et al. 2004; Luppi et al. 1996; Roggero et al. 2000; Zucca et al. 2000a, b). While an association between B. burgdorferi and primary cutaneous MALT lymphoma has been described in Europe, this has not been replicated in North American and Asian studies (Goodlad et al. 2000; Jelic and Filipovic-Ljeskovic 1999; Li et al. 2003; Wood et al. 2001). Immunoproliferative small intestinal disease is a lymphoma arising from small bowel MALT that is most commonly seen in the Middle and Far East, Mediterranean basin, and Africa (Lecuit et al. 2004). This geographic pattern of disease and the fact that reports of early stage disease response to antibiotics prompted a search for an infectious etiologic agent in its genesis. In seven patients who had responded to antimicrobial treatment, there was evidence of C. jejuni infection in four patients and H. pylori infection in no patients, thus establishing a potential link between C. jejuni infection and lymphomagenesis. The relative risk for patients with HCV infection to develop marginal zone lymphoma (particularly splenic and extranodal marginal zone lymphoma) is 2.5 times higher than the general population in a large intercontinental study (de Sanjose et al. 2008). Although this association is perhaps best described in patients with splenic marginal zone lymphoma, there is a high frequency of HCV infection in MALT lymphomas, and there have been reports of advanced MALT lymphomas of the salivary gland and intestines in patients with HCV infection that responded to treatment of the viral infection (Arcaini et al. 2004, 2009; Kelaidi et al. 2004; Svoboda et al. 2005). There is an association between patients presenting with a subcutaneous subtype of MALT lymphoma and HCV infection as well, with some patients responding to antiviral therapy (Paulli et al. 2010). In patients with MALT lymphoma, the incidence of HCV infection has been reported to be 35–43 %, most frequently in lymphomas of the skin, salivary glands, and orbit (Arcaini et al. 2007, 2009). Interestingly, these lymphomas frequently harbor the classical t(14;18) translocation joining the genes for BCL2 and the immunoglobulin heavy chain (Libra et al. 2004). The presence of HCV infection does not appear to influence the prognosis of the MALT lymphoma (Arcaini et al. 2007).
14.4 Clinical Presentation and Evaluation
14.4.1 Clinical Presentation
The clinical presentation of MALT lymphoma depends in large part on the site of disease. Gastric and intestinal MALT lymphomas may present with symptoms of dyspepsia and abdominal pain, sometimes with signs and symptoms of bowel obstruction but rarely with bleeding. These lymphomas are diagnosed on endoscopy with biopsies from multiple areas of endoscopically abnormal tissue as well as random sampling of macroscopically uninvolved mucosa. Lymphomas involving the small bowel may require a capsule video endoscopy for visualization. Involvement of the salivary and lacrimal glands, on the other hand, can result in Sjögren-like syndromes of dry eyes and mouth. MALT lymphomas involving the ocular adnexa typically present with painless conjunctival injection and photophobia, resembling allergic conjunctivitis. In the latter case, lesions are often bilateral and multifocal. Bronchus-associated lymphoid tissue (BALT) lymphoma involving the lungs and bronchi is a disease that most often affects older men (>60 years) (Fiche et al. 1995; Li et al. 1990). Unlike other MALT lymphomas, just over half of patients are symptomatic at diagnosis, with symptoms including cough, fever, and/or weight loss. This disease is often multifocal, spreading to other areas of the lungs and to other mucosal sites (Cordier et al. 1993). Other sites of disease often present with an obstructing mass. Some patients are diagnosed incidentally, either because of imaging studies or an exam of the eye or gastrointestinal track done for another reason or as part of an evaluation for a monoclonal gammopathy, which is present in approximately 25–35 % of MALT lymphoma patients; this feature is generally associated with plasmacytoid differentiation (Wohrer et al. 2004). B symptoms are rare in this disease (Armitage and Weisenburger 1998). Bone marrow involvement is present in a minority of patients, so cytopenias are rare, as is disease in the peripheral blood (Armitage and Weisenburger 1998).
14.4.2 Evaluation and Staging
The initial evaluation of a patient newly diagnosed with a MALT lymphoma should include a complete physical exam with attention to Waldeyer’s ring, including an assessment of performance status with either the Karnofsky performance scale or Eastern Cooperative Oncology Group performance status assessment. Laboratory evaluation should include a complete blood count with differential, comprehensive metabolic panel and liver function tests, lactate dehydrogenase, and an assessment of complete hepatitis B virus markers in patients who may be treated with rituximab. HCV testing may also be performed given its association with MALT lymphoma; a human immunodeficiency virus test is advised. Additional laboratory studies to consider include a β2-microglobulin, serum protein electrophoresis and immunofixation, and serum light chains. Staging is done with computed tomography (CT) scans of the chest, abdomen, and pelvis, as well as imaging of the neck, including the parotids and salivary glands, and orbits with CT or MRI. Although reports on the utility of positron emission tomography (PET) scans have been conflicting in this disease, with some demonstrating a lack of PET-avid signal in involved tissues and nodes and others suggesting that PET scans result in the upstaging of the disease at diagnosis, current recommendations of an International Harmonization Project in 2007 recommend against the use of PET scanning in the initial evaluation of lymphomas that are not routinely PET avid, including marginal zone lymphoma, based on a lack of sufficient evidence supporting its use (Beal et al. 2005; Fueger et al. 2009; Hoffmann et al. 1999; Juweid et al. 2007). A bone marrow biopsy should be considered for patients with multifocal disease, and an evaluation of the gastric mucosa is reasonable for all patients with non-gastric MALT lymphoma given the documented high rate of gastric involvement in these patients (Raderer et al. 2006).
Gastric and intestinal MALT lymphomas are staged by the Lugano staging system for gastrointestinal lymphomas (Table 14.1) (Rohatiner et al. 1994). By this system, stage I disease is limited to the gastrointestinal track, whereas stage II disease involves extraintestinal lymph nodes within the abdomen, and stage IV disease involves supradiaphragmatic nodal or disseminated extranodal tissue; there is no stage III disease. Endoscopic ultrasound has allowed for an estimate of the depth of infiltration into the gastric wall, which correlates with the extent of lymph node involvement and prognosis (Steinbach et al. 1999). Although the majority are localized at diagnosis, these lymphomas can spread to other parts of the gastrointestinal tract and the splenic marginal zone, perhaps due to H. pylori-activated T-cell-induced overexpression of the mucosal-homing integrin α4β7, whose ligand is expressed by these two tissues (Briskin et al. 1997; Du et al. 1997; Kraal et al. 1995). Staging for non-gastric MALT lymphoma is by the Ann Arbor staging system for lymphoma, which takes the extent of lymph node stations involvement as well as extranodal involvement into account when assigning a stage (Table 14.1) (Carbone et al. 1971; Lister et al. 1989).
Table 14.1
Staging MALT lymphoma
Ann Arbor staging for lymphoma | ||
Stage | Description | |
I | Involvement of a single lymph node region (I) or single extranodal site (IE) | |
II | Involvement of two or more lymph node regions or lymphatic structures on the same side of the diaphragm alone (II) or with involvement of limited, contiguous, extralymphatic organ or tissue (IIE) | |
III | Involvement of lymph node regions on both sides of the diaphragm (III), which may include the spleen (IIIS), or limited, contiguous, extralymphatic organ or tissue (IIIE), or both (IIIES) | |
IV | Diffuse or disseminated foci of involvement of one or more extralymphatic organs or tissues, with or without associated lymphatic involvement | |
All stages are further subdivided according to the absence (A) or presence (B) of systemic B symptoms including fevers, night sweats, and/or weight loss (>10 % of body weight over 6 months prior to diagnosis) | ||
Lugano staging system for gastric lymphomas | ||
Stage | Description | |
I | Tumor confined to the gastrointestinal tract | |
I1 | Infiltration limited to mucosa with or with submucosa | |
I2 | Infiltration of muscularis propria, subserosa, or serosa | |
II | Tumor extending into the abdomen from a primary gastrointestinal site | |
II1 | Local nodal extension | |
II2 | Distant nodal extension (para-aortic, para-caval, pelvic, inguinal) | |
IIE | Penetration of serosa to involve adjacent organs or tissues | |
IV | Disseminated extranodal disease or supradiaphragmatic involvement | |
14.4.3 Prognosis
Unlike for other non-Hodgkin lymphomas, there exists no specific prognostic scoring system for marginal zone or MALT lymphomas. The Follicular Lymphoma International Prognostic Index (FLIPI) was developed as a prognostic tool in follicular lymphoma and is often applied to other indolent lymphomas (Buske et al. 2006; Solal-Celigny et al. 2004). This index incorporates age (>60 years), Ann Arbor stage (III–IV), hemoglobin level (<12 g/dL), lactate dehydrogenase serum level (> upper limit of normal), and number of involved nodal areas (>4) into a 5-point scoring scale that corresponds to low (0–1 points), intermediate (2 points), and high (3 or more points) risk. A revised FLIPI has been developed, the FLIPI2, based on the results of a prospective study of almost 1,000 patients with newly diagnosed follicular lymphoma who underwent therapy that incorporates age (>60 years), bone marrow involvement, hemoglobin level (<12 g/dL), longest diameter of the largest involved lymph node (>6 cm), and a serum β2-microglobulin level (> upper limit of normal) into a 5-point scoring scale corresponding to low- (0 points), intermediate- (1–2 points), and high- (3 or more points) risk disease (Federico et al. 2009).
MALT lymphomas overall, however, have a good prognosis with a 5-year overall and failure-free survival of 81 % and 65 %, respectively (Nathwani et al. 1999). Patients with stage III or IV disease assessed before the era of rituximab and treated with cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) chemotherapy had a median overall survival of 5 years with median failure-free survival of 3 years (Fisher et al. 1995). In one retrospective series of a heterogeneous cohort of patients treated for MALT lymphoma, relapse rates approached 40 % at 4-year follow-up (Raderer et al. 2005). Non-gastric lymphomas had a higher rate of relapse than their gastric counterparts (48 vs. 22 %). In these patients, a high IPI and lymph node involvement has been associated with worse outcomes, whereas multiple mucosal sites were not (Thieblemont et al. 1997; Zucca et al. 2000a, b). Other series have shown that poor prognostic features in non-gastric MALT lymphomas include multiple MALT lymphoma sites, advanced stage disease, bone marrow and nodal involvement, and MALT lymphomas outside the skin and ocular adnexa (Arcaini et al. 2006). Primary cutaneous marginal zone lymphomas are particularly indolent and unlikely to disseminate with 5-year survival rates of 98–100 % (Hoefnagel et al. 2005). The presence of systemic symptoms in BALT lymphomas has been associated with a poorer prognosis (Cordier et al. 1993).
In gastric MALT lymphomas, the presence of a t(11;18) translocation is associated with an increased risk of disseminated disease, whereas trisomy 18 is associated with a risk of advanced stage disease in extragastric MALT lymphoma (Raderer et al. 2006). However, whereas t(11;18)-positive MALT lymphomas rarely are associated with additional clonal aberrations, a majority of t(11;18)-negative MALT lymphomas have allelic imbalances that are also present in gastric DLBCL (Starostik et al. 2000; Starostik et al. 2002). Thus, t(11;18)-positive lymphomas appear to be genetically stable and therefore at lower risk for transformation into a more aggressive DLBCL. Instead, certain genetic alterations appear to increase the risk of histologic transformation into an aggressive lymphoma including loss or deletion of TP53, hypermethylation or deletion of CDKN2A, and other chromosomal gains or losses (Du et al. 1995; Martinez-Delgado et al. 1997; Neumeister et al. 1997). Aberrant expression of CD5 detected by flow cytometry or immunohistochemistry is likewise associated with a more aggressive phenotype, as is high-grade histology (de Jong et al. 1997, 2000; Wenzel et al. 2001). A grading system for MALT lymphoma has been devised that assigns a letter grade (A to D) based on the number of blast or blast clusters seen histologically, ranging from <5 % blasts in clusters of up to ten cells to pure DLBCL without a low-grade component (de Jong et al. 1997). Gastric lymphomas that do not respond to H. pylori eradication are enriched for higher-grade histology, although many patients with high-grade histology do respond to antibiotic therapy (Bayerdorffer et al. 1995; Chen et al. 2001). In one series of 16 patients with stage I–II H. pylori-positive DLBCL of the stomach, half achieved a complete remission with antibiotic therapy alone, and another 13 % had a partial remission convert to a complete remission after the addition of rituximab monotherapy (Govi et al. 2011b). After 53-month follow-up, nine of the ten complete responders were still in remission. Grade similarly correlated with prognosis in a small number of patients with MALT lymphoma of the thyroid gland treated in the pre-rituximab era; all patients with low-grade lesions were alive at a median follow-up of 26 months, whereas patients with high-grade lesions appeared to do worse than patients with pure DLBCL of the thyroid gland with a low 5-year overall survival rate of 25 % owing to a higher rate of more advanced stage disease at diagnosis (Skacel et al. 2000).
14.5 Management
Management of MALT lymphoma depends both on stage and site of disease. As an indolent lymphoma with a long overall survival, close observation at diagnosis until the development of signs, symptoms, or organ function impairment as a result of the disease is appropriate for patients with more advanced stage disease. An exception is patients with advanced stage MALT lymphoma and concomitant HCV infection; a trial of anti-HCV antiviral therapy in these patients may result in regression of their lymphoma. For patients with early stage and localized disease, however, treatment with local therapies such as radiation and at times surgery, or treatment with antibiotics for H. pylori-positive gastric MALT lymphoma, has been associated with high response rates and durable responses, some of which may represent cures. Treatment of symptomatic or organ impairing relapsed, refractory, or advanced stage disease is similar to approaches used in follicular lymphoma with chemotherapy, immunotherapy, or chemoimmunotherapy. The general treatment strategies as well as the treatment strategies employed for specific situations will be outlined in detail in this section (Fig. 14.2).
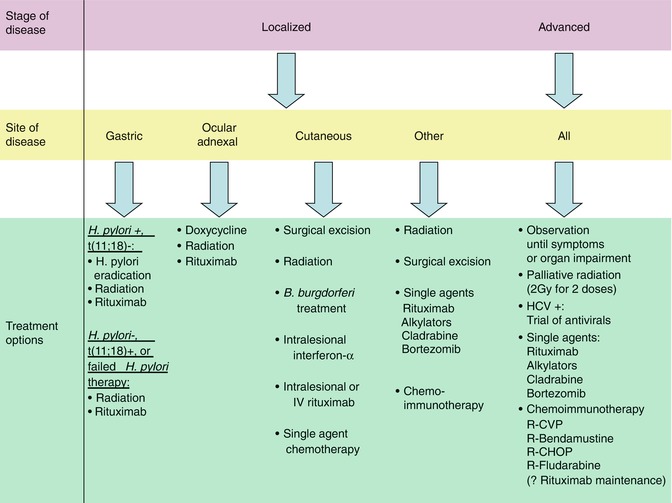

Fig. 14.2
Treatment algorithm for MALT lymphoma (Treatment options for localized and advanced stage MALT lymphoma. In general, the order of therapies in each category parallels the order preference for treatment in clinical practice. Abbreviations: R-CVP rituximab; cyclophosphamide; vincristine; prednisone, R-CHOP rituximab; cyclophosphamide; doxorubicin; vincristine; prednisone, HCV Hepatitis C virus)
14.5.1 Early Stage Gastric MALT Lymphoma
Early stage or localized MALT lymphoma of the stomach is an indolent disease that is often associated with H. pylori infection. Eradication of H. pylori is effective treatment for many patients with good long-term disease control and overall survival and is recommended for most patients with H. pylori-positive gastric MALT lymphomas that do not harbor a t(11;18) translocation (Bayerdorffer et al. 1995; Chen et al. 2005; Fischbach et al. 2004; Neubauer et al. 1997; Roggero et al. 1995; Stathis et al. 2009; Steinbach et al. 1999; Wotherspoon et al. 1993; Wundisch et al. 2005). In patients with H. pylori-negative lymphomas, MALT lymphomas with a t(11;18) translocation, or lymphomas that fail to respond to H. pylori therapy, radiation therapy is the preferred treatment modality (Hitchcock et al. 2002; Schechter et al. 1998; Tomita et al. 2009; Tsang et al. 2001, 2003). Chemotherapy, immunotherapy, or chemoimmunotherapy is active in this disease but is generally reserved for patients with relapsed or refractory disease to antibiotic or radiation therapy or patients with more advanced stage or aggressive disease (Conconi et al. 2003, 2011; Hammel et al. 1995; Jager et al. 2002; Martinelli et al. 2005; Nakamura et al. 2005; Raderer et al. 2003).
14.5.1.1 Eradication of H. pylori
Based on the epidemiologic and preclinical data supporting a causal role of H. pylori infection in the pathogenesis of gastrointestinal MALT lymphoma, the effect of H. pylori therapies on these lymphomas was investigated. The first report of six patients treated as such was published in 1993: all six patients had complete eradication of their H. pylori, and all but one had complete regression of their lymphoma (Wotherspoon et al. 1993). A number of additional patient series have since been reported, with complete response rates ranging from 50 to 83 % and 7-year freedom from relapse rates approaching 78 % (Bayerdorffer et al. 1995; Chen et al. 2005; Fischbach et al. 2004; Neubauer et al. 1997; Roggero et al. 1995; Stathis et al. 2009; Steinbach et al. 1999; Wundisch et al. 2005). Perhaps the only randomized trial in MALT lymphoma, the LY03 trial, randomized patients to adjuvant chlorambucil versus observation following H. pylori treatment for early stage gastric MALT lymphoma (Hancock et al. 2009). This trial demonstrated no benefit of adjuvant chlorambucil over observation with respect to relapse rate, progression-free survival, or overall survival.
Modern H. pylori treatment regimens include a combination of two or three antibiotics, often clarithromycin and either amoxicillin or metronidazole, with a proton pump inhibitor, with or without bismuth salicylate for a total of 10–14 days; approximately 20 % of patients will require a second course of therapy for complete eradication of the organism. The median time from treatment to the histologic complete regression of the lymphoma ranges from 6 to 36 months. Posttreatment evaluation has not been systematically studied, but general recommendations include a urea breath test at 4–8 weeks after treatment to confirm eradication of the organism and once eradicated, endoscopic biopsies every 1–3 months until a histologic complete response is documented. Endoscopic surveillance is then every 6 months for up to 2 years or as indicated by symptoms.
Approximately 20–30 % of patients will not respond to H. pylori therapies within 12–18 months of treatment. Depth of invasion in one study correlated inversely with the likelihood of response, with only 42 % of patients with lymphomas extending to the muscularis, subserosa, or perigastric lymph nodes achieving a complete response to H. pylori treatment (Steinbach et al. 1999). Other studies have also shown that lymph node involvement was associated with a decreased response rate (Ruskone-Fourmestraux et al. 2001). The t(11;18) translocation predicts for poor response to H. pylori-directed therapies as well (Alpen et al. 2000). In addition, approximately 10–20 % of patients who do achieve a complete response will relapse (Neubauer et al. 1997; Stathis et al. 2009). Many of these relapses occur in the context of persistently negative H. pylori studies, pointing towards the development of a self-sustaining antigen-independent lymphoma clone in these patients (Fischbach et al. 2004; Neubauer et al. 1997). Molecular evidence of B-cell clonality often persists following H. pylori-directed therapies, and while the clinical significance of this is unknown, it does not appear to correlate with clinical relapse with reasonable follow-up (Bertoni et al. 2002; Fischbach et al. 2002; Montalban et al. 2005; Thiede et al. 2001). There is evidence for clonal instability in this setting, with ongoing somatic hypermutation and antigen selection evidenced by immunoglobulin heavy chain sequencing (Thiede et al. 1998). The persistence of molecular evidence of disease does support the notion that eradication of H. pylori results in suppression but not elimination of the lymphoma clone. Longer follow-up, then, is necessary to determine if antimicrobial-directed therapy is a curative option in this disease.
14.5.1.2 Radiation Therapy
In the early 1990s, gastrectomy and surgical resection were the preferred treatment modality for patients with early stage gastric MALT lymphoma (Bozzetti et al. 1993). The issue with partial gastrectomy alone was mostly a high rate of relapse given the multifocal nature of this disease, and total gastrectomy was associated with a high degree of morbidity (Montalban et al. 1995). With the efficacy and tolerability of less invasive treatments, like H. pylori therapies and radiation, surgery plays a limited, if any, role in the treatment of MALT lymphoma. Radiation therapy, then, is the treatment of choice for patients with H. pylori-negative gastric MALT lymphoma, gastric MALT lymphomas with a t(11;18) translocation, or gastric MALT lymphomas that have failed an antimicrobial approach.
The first series of 17 patients treated with gastric MALT lymphoma treated with radiation therapy alone at a dose of 30 Gy over 4 weeks resulted in a complete remission rate of 100 % and 2-year event-free survival of 100 %, even in patients who had evidence of perigastric lymph node involvement (Schechter et al. 1998). The use of radiation for the treatment of early stage MALT lymphoma in series of patients involving multiple tissue types demonstrates excellent local control with local relapses observed in 0–5 % of patients and good long-term disease control with 5-year progression-free and overall survivals of 75–82 % and 93–97 %, respectively (Hitchcock et al. 2002; Tomita et al. 2009; Tsang et al. 2001, 2003). Radiation therapy to the stomach is well tolerated, and the risk of secondary malignancies is low.
14.5.1.3 Chemotherapy and Immunotherapy
The use of chemotherapy and/or immunotherapy in gastric MALT lymphoma is typically limited to patients who fail antimicrobial-directed therapies or for patients with locally advanced or advanced stage disease. For early and more advanced stage disease, agents that have been used and reported include single-agent therapy with alkylating agents such as chlorambucil or cyclophosphamide; purine analogues such as cladribine, bortezomib, and rituximab; and occasionally multi-agent anthracycline-based chemotherapy for younger patients with more aggressive disease.
The use of single-agent, continuous, low-dose oral chlorambucil or cyclophosphamide in 24 patients with early or advanced stage disease yielded complete response rates of 75 % and a relapse rate of 21 % during the 8-year follow-up (Hammel et al. 1995). Similar to resistance to H. pylori therapies, the t(11;18) translocation was associated with alkylator resistance in this study. While these outcomes appear to be inferior to those observed following radiation or H. pylori eradication, the use of single-agent oral alkylating agents as second-line treatment after a failure of H. pylori treatment was equivalent to radiation therapy in one study of small patient numbers (Nakamura et al. 2005). As mentioned above, the LY03 trial was a randomized trial of observation versus adjuvant chlorambucil in patients with localized gastric MALT lymphoma treated with H. pylori therapies and showed no benefit with the addition of systemic therapy, even in patients with molecular evidence of disease following eradication therapy (Bertoni et al. 2002; Hancock et al. 2009).
The purine analogue cladribine has been investigated as first-line therapy for patients with stage I–IV gastric and non-gastric MALT lymphoma. All 19 patients with gastric MALT lymphoma treated achieved a complete remission, including those with early stage disease that had failed H. pylori eradication, but three of these patients relapsed within 32 months (Jager et al. 2002). Although all seven patients with non-gastric MALT, all of whom had stage II–IV disease, had a disease response, only 43 % of them had a complete response. This data suggests that cladribine is active in MALT lymphoma that is either refractory to initial therapy or more advanced stage at presentation.
The introduction of the highly efficacious anti-CD20 antibody rituximab for the treatment of mature B-cell malignancies has made this an attractive therapy for gastric MALT lymphomas, especially those that are H. pylori negative or refractory to H. pylori eradication. The first report of nine patients with advanced MALT lymphoma treated with single-agent rituximab was disappointing; however, with responses seen in five patients, three of whom achieved a complete response (Raderer et al. 2003). A larger, prospective cohort of 35 patients with stage I–IV MALT lymphoma (15 gastric, 10 non-gastric) who were either chemotherapy naïve or who had progressed following chemotherapy was treated with single-agent rituximab; the overall response rate in this population was 73 % and was better for chemotherapy naïve patients than for previously treated patients (87 vs. 45 %) (Conconi et al. 2003). Duration of response was short, however, with 36 % of responders progressing at a median of 10.5 months. A series of 27 patients with gastric MALT lymphoma that was either relapsed/refractory to, or not otherwise eligible for, H. pylori eradication was treated with rituximab with slightly more promising results: the overall response rate was 77 % with a complete response rate of 46 %, and only two patients had relapsed at a median follow-up of 33 months (Martinelli et al. 2005). Interestingly, this and cladribine are the first two treatment modalities for which the presence of the t(11;18) translocation did not predict for lack of response or relapse (Martinelli et al. 2005; Streubel et al. 2004). Rituximab, then, is an attractive option for patients who have localized disease relapse following H. pylori eradication and/or radiation therapy, or for whom either of these treatment modalities are not options. Additionally, it is an attractive and effective option for advanced disease as will be outlined later in this section. Combination chemoimmunotherapy has been investigated, and the combination of rituximab and fludarabine for patients with systemically untreated gastric and non-gastric MALT lymphoma of any stage does appear to improve response rates and response duration with response rates of 85–100 % and 2–3-year progression-free survival of 80–100 % (Brown et al. 2009; Salar et al. 2009). This comes at the expense of significantly greater hematologic and infectious toxicity, however, that is prohibitive in these patients. There is an ongoing phase III three arm randomized trial comparing rituximab alone, chlorambucil alone, and rituximab and chlorambucil in combination in patients with any stage MALT lymphoma having no prior therapy or following antibiotic or radiation therapy.
Most recently the proteasome inhibitor bortezomib, which is known to inhibit the NF-κB pathway, has been studied in relapsed or refractory MALT lymphoma based on the understanding of the importance of NF-κB signaling in the pathogenesis of these diseases (Conconi et al. 2011). Thirty-two patients with both gastric and non-gastric, early stage and advanced stage, and relapsed and refractory MALT lymphoma received bortezomib monotherapy with an overall response rate of 48 %; an additional 36 % of patients had stable disease after a median follow-up of 24 months. Other options for the treatment of relapsed or refractory disease mirror chemoimmunotherapy regimens used in follicular lymphoma and include combination therapies with rituximab and bendamustine or cyclophosphamide, vincristine, and prednisone (R-CVP) or anthracycline-containing regimens like rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for younger patients with more aggressive disease. These multi-agent regimens will be discussed in more detail in Sect. 14.5.3 but are recommended for more aggressive and advanced stages of disease.
14.5.2 Early Stage Non-gastric MALT Lymphoma
Treatment of early stage non-gastric MALT lymphomas follows many of the same paradigms as for early stage gastric MALT lymphomas in Sect. 14.5.1, except that there is no association between a particular infectious organism and these lymphomas that is as strong or penetrant as the association between H. pylori and gastric MALT lymphoma. MALT lymphomas outside the stomach are also positive for H. pylori, in up to 45 % of cases in one series, but H. pylori treatment only led to regression of one colonic tumor in 16 such patients (Grunberger et al. 2006). Although antibiotic approaches against B. burgdorferi for cutaneous lymphomas and C. jejuni for intestinal lymphomas have been investigated and successful in some cases, the treatment of choice for most localized, early stage disease is radiation therapy (Hitchcock et al. 2002; Isobe et al. 2007; Tomita et al. 2009; Tsang et al. 2001, 2003). The use of doxycycline for treatment of C. psittaci-associated MALT lymphoma of the ocular adnexa has been resulted in high response rates in certain patient populations and is a reasonable upfront treatment option, in addition to radiation therapy (Govi et al. 2011a). For many of these lymphomas, doses of 24–30 Gy are sufficient and associated with minimal toxicity. One prospective series of 37 patients with stage IE non-gastric MALT lymphoma treated with a median of 30.6 Gy of radiation reported a 92 % complete response rate, with a 3-year progression-free and overall survival of 92 % and 100 %, respectively (Isobe et al. 2007). Many of the relapses involved the contralateral paired organ with nearly 100 % local control (Tsang et al. 2001, 2003). Surgical resection is appropriate for tumors that are not amenable to radiation and has been reported in patients with lymphomas involving the salivary glands, thyroid, skin, breasts, lung, genitourinary tract, and dura (Ambrosetti et al. 2004; Cerroni et al. 1997a; Ferraro et al. 2000; Gogas et al. 2002; Kees et al. 2005; Kempton et al. 1997; Zinzani et al. 2003). Surgery may be performed before the diagnosis of MALT lymphoma is known, and if complete excision is achieved, these patients should be observed without further treatment; if margins remain positive though, adjuvant radiation should be administered when feasible. Improving on outcomes following radiation therapy with adjuvant chemotherapy has not been beneficial: the addition of adjuvant anthracycline-based chemotherapy following radiation for stage IE orbital MALT lymphomas did not improve outcomes, whereas such adjuvant chemotherapy in stage III–IV non-gastric MALT lymphomas improved only the complete response rate but not progression-free survival (Aviles et al. 2006; Oh et al. 2007). Chemotherapy and/or immunotherapy approaches as were outlined for early stage gastric MALT lymphomas have been studied in non-gastric MALT lymphomas as well and are appropriate for cases of relapsed or refractory disease to local therapies like surgery or radiation, multifocal disease that is not amenable to local therapies, or more aggressive or advanced stage disease (Conconi et al. 2003, 2011; Hammel et al. 1995; Jager et al. 2002; Raderer et al. 2003). Although perhaps less effective for non-gastric compared to gastric MALT lymphomas, rituximab is a reasonable first option for some of these patients. Single-agent alkylating agents like oral cyclophosphamide or chlorambucil or purine analogues like cladribine have activity in these diseases as well (Conconi et al. 2003, 2011; Hammel et al. 1995; Jager et al. 2002). The response rates and duration of responses seen with these single agents can be low, making combination chemoimmunotherapy with regimens like rituximab and bendamustine or rituximab and cyclophosphamide, vincristine, and prednisone more appropriate for some patients. For patients who have no signs or symptoms related to their disease and who have either relapsed following local therapies or are not candidates for local therapies, observation with close follow-up is the preferred option (Ardeshna et al. 2003). The remainder of this section will outline the approaches that have been investigated for MALT lymphomas of specific disease sites: ocular adnexa, thyroid, breast, skin, lung, dura, and genitourinary tract.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree