William M. McCormack, Michael H. Augenbraun Keywords atrophic vaginitis; bacterial vaginosis; Candida vaginitis; candidiasis; cervicitis; Chlamydia trachomatis; desquamative; estrogen-deficiency vaginitis; Gardnerella vaginalis; gonorrhea; inflammatory vaginitis; metronidazole; tinidazole; Trichomonas vaginalis; trichomoniasis; vaginitis; vestibulitis; vulvitis
Vulvovaginitis and Cervicitis
Vulvovaginal symptoms are common and frequently result in encounters of patients with the health care system, including use of folk remedies, purchase of over-the-counter (OTC) pharmaceuticals, and presentation to health care providers. In one survey1 of female college graduates with a median age of about 30 years, almost 70% reported at least one lifetime physician-diagnosed and treated vaginal yeast infection; in addition, 29% had been treated for bacterial vaginosis (BV) and 26% for trichomoniasis. More than 20% of the women had been treated for a vaginal yeast infection within the past year.
Genitourinary symptoms in women tend to lack specificity. All women have vaginal secretions, but their ability to discriminate normal from abnormal secretions is often imprecise. Similarly, vulvar discomfort is fairly common and may result from a variety of infectious and noninfectious causes. This chapter discusses vaginal discharge and vulvitis separately, with the understanding that there is considerable overlap between these syndromes and that a patient whose symptoms appear to represent one syndrome may ultimately have a quite different diagnosis.
Vaginal Secretions
The normal vaginal secretions are a physiologically important biomass. Vaginal cells contain glycogen and are continually shed into the lumen of the vagina. As the cells autolyze, glycogen depolymerizes to glucose, which serves as an energy source for bacteria known as lactobacilli. Data reported by Ravel and associates demonstrated that the vaginal bacterial communities of asymptomatic North American women clustered into five groups: four were dominated by Lactobacillus crispatus, L. jensenii, L. iners, or L. gasseri, whereas the fifth had lower proportions of lactobacilli and higher proportions of strictly anaerobic organisms.2
Lactobacilli metabolize glucose to lactic acid, which results in a normal vaginal pH of about 4.0.
In the studies of Ravel and associates,2 the vaginal communities of women whose flora was dominated by L. jensenii and those with higher proportions of strictly anaerobic bacteria had higher mean pH levels than the other groups. In addition, blacks and Hispanics had higher mean vaginal pH levels than whites and Asians.
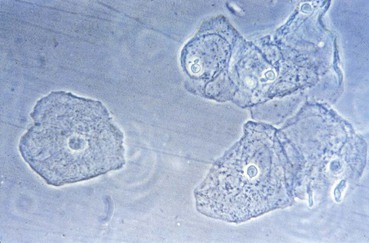
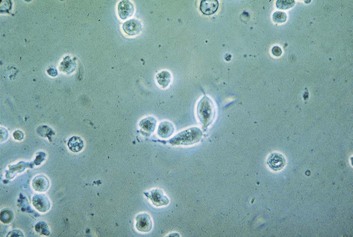
Although the vagina is not sterile and many species of bacteria can be isolated from vaginal specimens from healthy women, lactobacilli often dominate the vaginal microbiota. Indeed, the predominant cells in the vaginal secretions of most normal women are lactobacilli and vaginal epithelial cells (Fig. 110-1).
In addition to producing lactic acid, lactobacilli may also produce hydrogen peroxide, which is bactericidal alone and highly bactericidal in combination with physiologic amounts of myeloperoxidase and chloride.3 Loss of the normal Lactobacillus-dominated vaginal microbiota increases the likelihood of exogenous infection after exposure to sexually transmitted pathogens,4 as well as the risk for endogenous infection in association with pregnancy and gynecologic surgery.5
Therefore, the normal vaginal secretions are a heterogeneous suspension of vaginal epithelial cells and lactobacilli in fluid that emanates from the cervix and vaginal walls (Fig. 110-2). The secretions have a pH of 3.5 to 4.6, are odorless, and do not cause itching or irritation. The normal secretions are clumpy and tend to remain in the vagina, although in some women egress of normal secretions may require use of a perineal pad. Characteristics of normal vaginal secretions are listed in Table 110-1.
An appreciation of what is normal is important in dealing with patients who report an abnormal discharge. A significant minority of such patients have normal secretions. These women may have a large volume of normal secretions; relaxation of the introitus after childbirth that allows outflow of normal volumes of normal secretions; or heightened awareness of a normal amount of physiologic secretions, often as a result of other genitourinary symptoms such as vulvar discomfort. The volume of normal secretions may increase during pregnancy and during use of hormonal contraceptive agents.
Patients with a normal or physiologic vaginal discharge usually have a history of multiple self-directed or clinician-directed treatments with a bewildering array of topical and systemic antibacterial and antifungal agents. Recognition of the normalcy of the secretions is important so that the patient can be reassured that her secretions are normal and so that diagnostic and therapeutic attention can be focused on associated conditions, if any, that may be present.
Important characteristics of patients who present with normal vaginal secretions are listed in Table 110-2. Notable characteristics include chronicity, absence of odor, absence of vulvar discomfort (unless there is unrelated vulvitis), many unsuccessful visits to a variety of different health care providers, and many ineffective treatments.
On close questioning, a number of these patients do not have sufficient external flow of vaginal secretions to stain their clothing or to require use of a perineal pad. They appreciate what they perceive to be abnormal secretions by extracting normal secretions with use of their index finger. This finger test is an important historical clue that the woman has normal secretions.
In addition to normal secretions, the differential diagnosis of vaginal discharge primarily includes three infections (trichomoniasis, vulvovaginal candidiasis, and BV); an idiopathic condition known as desquamative inflammatory vaginitis (DIV); cervicitis (both infectious and noninfectious); and vulvovaginitis associated with estrogen deficiency (Table 110-3).
Approach to the Patient
History
The etiologic diagnosis of vaginitis depends on a careful evaluation of the history, a physical examination, and immediate laboratory tests. Historical features are relatively nonspecific,6 but they may direct clinical suspicion toward certain causes. If at all possible, the history should be obtained with the patient in the sitting position and before she has disrobed.
The medical history (Table 110-4) should include all of the usual gynecologic parameters, including menstrual history, pregnancies, contraception, sexual preference, past and current sexual relationships, and prior genitourinary infections. Additionally, the patient should be asked about underlying medical conditions such as allergies, diabetes, malignancies, and immunodeficiency syndromes (primarily human immunodeficiency virus [HIV] infection) that might be associated with or influence vulvovaginal disease.
Age
Neonates can acquire trichomonal or candidal vulvovaginitis during passage through an infected birth canal, which would argue for treating these infections in pregnant women before term. Neonatal vaginal thrush responds promptly to topical antifungal medications. Neonatal trichomoniasis can be treated with metronidazole. After the neonatal period, a vaginal discharge is abnormal and should prompt a thorough examination. Vaginal candidiasis is rare in prepubescent girls. Prepubescent vaginal epithelium is thin, and the entire vagina is susceptible to infection with Neisseria gonorrhoeae. Gonococcal vulvovaginitis often causes profuse vaginal discharge. The diagnosis of a sexually transmitted disease in a young girl should raise the suspicion of child abuse, although some agents have been transmitted to children in the absence of frank sexual contact.7 Patients in the sexually active years are more likely to have physiologic secretions or infectious vaginitis. Postmenopausal women are more likely to have atrophic vaginitis.
Mode of Onset
An abrupt and identifiable time of onset of symptoms suggests infection. Vaginal discharge associated with neoplasia, estrogen depletion, or a foreign body often has a subacute onset, with symptoms progressing over a period of weeks.
Quantity of Discharge
The amount of discharge is highly variable in all conditions. Patients with vulvovaginal candidiasis often have scanty discharge or no discharge at all. Atrophic discharges are commonly scanty unless infection has supervened.
External Irritation
Physiologic discharge is rarely associated with vulvar or perineal discomfort. Pruritus with a scant or absent discharge is frequently seen in candidiasis. External discomfort is an infrequent complaint in BV. Severe episodic perineal pain that sometimes prevents urination suggests herpes simplex virus infection, which affects the labia but usually spares the vagina. Chronic discomfort (often interfering with sexual activity) should prompt consideration of a noninfectious vulvitis such as vulvar vestibulitis.
Odor
Vaginal odor in the absence of other symptoms is the initial complaint in many cases of BV. A feculent odor may accompany anaerobic superinfection of genital lesions, or it may be noted in the presence of a foreign body or enterovaginal fistula.
Abdominal Pain
Abdominal discomfort is rare in uncomplicated vulvovaginitis, except for some cases of trichomoniasis. Women who complain of abdominal pain should be examined for evidence of urinary tract infection and pelvic inflammatory disease.
Sexual History
Exposure to a new sexual partner increases the likelihood of sexually transmitted disease. A history of genital symptoms in a sexual partner is helpful diagnostically. The commencement of oral contraceptive use may be associated with increased physiologic discharge.
Other Diseases
Diabetes mellitus, acquired immunodeficiency syndrome (AIDS), malignancy and the treatment thereof, and possibly hypoparathyroidism increase the risk for candidal vaginitis. Diseases known to impair host defenses may predispose to otherwise rare infections. Drugs used in the treatment of other diseases may predispose to vaginal infection.
Medications
Systemic or local medications may influence vaginal infection. Antibiotics that are active against the normal bacterial microbiota of the vagina predispose to candidal vaginitis.8 Patients who are taking corticosteroids or oral contraceptives are at increased risk for development of vulvovaginal candidiasis. Local medications, including vaginal douches, rarely produce a chemical vaginitis; douching immediately before examination makes diagnosis difficult.
Examination
If the patient has not had recent medical care, a general physical examination can be performed. At the least, the patient’s breasts should be examined if she has not had a professional breast examination during the past year. Similarly, a mammogram should be ordered if the patient’s age or history dictate that one is indicated.
With the patient supine on the examining table, the pubic hair should be examined for the presence of crab lice or nits. The inguinofemoral areas should be palpated for adenopathy. Suprapubic and lower abdominal tenderness or masses can be sought by palpation.
The gynecologic examination (Table 110-5) requires adequate light. Magnification is helpful and is best provided by a colposcope. Providing the patient with a mirror allows her to participate in the examination and is useful for clarifying the location of symptoms as well as demonstrating important findings to the patient.
With the patient in the lithotomy position, the external genitalia should be carefully inspected. The patient should be asked to point to any areas of external itching, irritation, or other discomfort. Localization of discomfort to vulvar skin or vestibular mucosa provides useful information. The appearance of the vulvar skin and vestibular mucous membranes should be noted, with careful attention to ulcers or other discontinuity of the skin or mucosa, mucosal erythema, and visible secretions. Diffuse perineal erythema may accompany candidiasis. Diffuse reddening with small satellite lesions, usually papular or papulopustular, suggests candidiasis. The degree of perineal irritation is quite variable with all infections, but severe perivaginal irritation is uncommon with BV. Labial edema may accompany severe irritation, especially in vulvovaginal candidiasis.
Careful examination of all the extravaginal surfaces may reveal lesions of genital herpes, syphilis, condyloma acuminatum, molluscum contagiosum, scabies, or vulvar vestibulitis.
By spreading the labia with the gloved hand, one may examine the urethral meatus. The urethra may be gently stripped with a finger placed inside the vagina. Urethral discharge is not a common finding, but, if delivered, such material should be examined microscopically and cultured. The introitus and the internal surfaces of the labia minora should be examined for lesions. Vaginal discharge is sometimes observed on the labia or flowing onto the perineum. Such copious discharge is usually associated with trichomoniasis or BV but may accompany other infections.
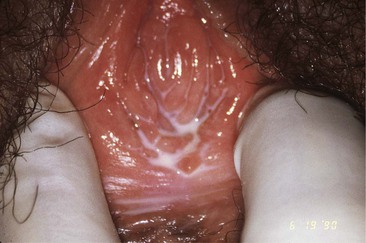
A speculum is then inserted to expose the cervix and vaginal mucosa. A small-sized speculum is adequate for most patients and minimizes discomfort for patients who have introital lesions. A small amount of lubricant facilitates insertion without compromising the quality of the microbiologic samples to be collected. The vaginal and cervical mucosa should be inspected with attention to erythema and lesions. An ectropion, if present, should be noted (Fig. 110-3). The vaginal secretions should be described, as should any secretions emanating from the endocervical canal or from an ectropion.
Specimens obtained during the examination are listed in Table 110-6. Vaginal pH should be measured. A sample of vaginal material should be collected from the lateral vaginal wall with use of a cotton-tipped applicator. Care should be taken to avoid contamination with endocervical secretions. The collected vaginal secretions are applied to a strip of pH paper and compared with the standard chart provided by the manufacturer (Fig. 110-4).
A sample of vaginal secretions is then examined. The specimen may be prepared in several ways. A swab of vaginal secretions may be agitated in a tube containing about 0.5 mL of normal saline to form a suspension. Alternatively, a plastic transfer pipette may be used to introduce 2 to 3 mL of normal saline solution into the posterior fornix. The saline solution is mixed with the vaginal secretions by aspiration and reaspiration of the solution, and the resultant suspension of vaginal material is placed into a small tube.
The suspension is then examined for odor (whiff test) by placing a drop on a microscope slide, adding a drop of 10% potassium hydroxide (KOH), and smelling the resultant mixture. Normal secretions have no odor. A fishy odor is indicative of BV.
A drop of the suspension of vaginal material is placed on a microscope slide, and a coverslip is added. This wet mount can be examined under high power with a bright-field microscope. Phase-contrast microscopy provides an excellent means of evaluating vaginal wet mounts. The relative numbers of epithelial cells and polymorphonuclear neutrophils (PMNs) should be noted. Because PMNs are present in physiologic endocervical discharge that collects in the vagina,9 small numbers of PMNs may be observed in the vaginal material recovered from healthy women. A finding of more PMNs than epithelial cells in a vaginal wet preparation should raise suspicion for cervical or vaginal inflammation. Observation of relatively few PMNs does not rule out vaginal infection, however. Vaginal candidiasis can produce a discharge that contains only small numbers of PMNs. The relative absence of PMNs is characteristic of the discharge of BV.10 In fact, the finding of many PMNs in the vaginal discharge of a patient with BV should prompt a search for simultaneous infection, such as trichomoniasis, gonorrhea, or chlamydial cervicitis. Pseudohyphae suggest vaginal candidiasis, but often only moderate or even very small numbers of yeast cells are seen in this condition. Indeed, some patients with vulvovaginal candidiasis have organisms identified only by culture. The wet preparation should be scanned for motile trichomonads.
Normal squamous epithelial cells have transparent cytoplasm and small nuclei. Immature (parabasal) cells are smaller and have larger nuclei. Epithelial cells covered with tiny coccobacillary forms are called clue cells and are associated with BV. Clue cells are best recognized by observing the edges of epithelial cells, which may be obscured by the adherent coccobacilli. Some cells are so heavily encrusted that the nuclei are obscured. Trichomonads are best recognized by their characteristic twitching motility. The flagellae and undulating membrane may be observed by careful focusing of the microscope and adjustment of the light source. Trichomonad motility may be improved by gentle warming of the preparation. The wet mount is negative in about 30% of the women with trichomoniasis (see Chapter 282), so a negative wet mount does not rule out this infection, particularly in asymptomatic women.
The bacterial microbiota can be assessed on the wet mount. Normal vaginal microbiota consists of a sparse population of bacilli. In BV, the predominant organisms are tiny coccobacilli. Spermatozoa may be observed as long as 10 days after the last coitus, but motile sperm suggest sexual contact within the preceding 24 hours.11
Combining a drop of 10% KOH with the vaginal material on a microscope slide and applying a coverslip destroys cellular elements but leaves the bacteria and fungi unscathed. The KOH preparation cannot be used for microscopic diagnosis of trichomoniasis or BV.
A Gram stain of vaginal material is somewhat less useful than the wet mount for differential diagnosis. Although Candida spp. are readily recognized on the Gram-stained smear, trichomonads are difficult to identify. Normal vaginal flora consists primarily of gram-positive bacilli, which are mostly lactobacilli. In BV, the normal flora is replaced by sheets of gram-variable coccobacilli, which often overlie the surface of epithelial cells. Women with vulvovaginal candidiasis sometimes have large numbers of budding yeasts and pseudohyphae. The Gram stain is negative in many women from whom Candida can be cultured.12
Material recovered from the endocervix can be Gram stained. Normal cervical discharge usually contains moderate numbers of PMNs, and their presence is not necessarily an indication of infection.9 The presence of large numbers of PMNs suggests cervicitis. Gram-negative, intracellular diplococci accurately diagnose gonorrhea (see Chapter 214), but extracellular diplococci are less predictive. The cervical Gram stain is positive in only about 60% of women with cervical gonorrhea. Therefore, a negative Gram stain does not rule out this infection.12
Before the speculum is removed, specimens are obtained for examination for N. gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis with use of culture or nonculture methods such as nucleic acid amplification tests. Vaginal cultures for yeast are useful to exclude fungal infection, for which the wet preparation is insensitive.13 Trichomonads can be sought by inoculating selective liquid media such as modified Diamond’s medium if trichomoniasis is a diagnostic possibility.14 Finally, a cervical cytologic smear should be obtained if such an examination cannot be documented within the currently recommended time frame. Relying on the patient’s history of cervical cytologic examinations is risky, because some women assume that all gynecologic examinations include a Papanicolaou (Pap) smear.
Bimanual examination for adnexal tenderness and masses should be a part of the evaluation. Adnexal tenderness is sufficiently uncommon with local vaginal infections that its presence suggests salpingitis.
Trichomoniasis
Etiology and Pathogenesis
Trichomoniasis is caused by the protozoan Trichomonas vaginalis. It is a classic exogenous sexually transmitted infection, like gonorrhea and chlamydial infection. The organism is not normally present in the vagina. Transmission almost always occurs through sexual contact. After an incubation period of a few days, patients develop a purulent discharge associated with varying degrees of vulvar irritation, dysuria, and dyspareunia (Table 110-7). An abnormal odor is often present, usually signifying concomitant BV.
Diagnosis
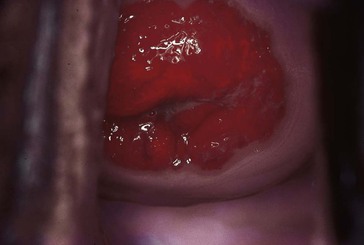
Examination is notable for vulvar, vestibular, and vaginal erythema and a purulent vaginal discharge (Fig. 110-5). A minority of patients manifest characteristic mucosal capillary dilation, which gives the mucosa a strawberry appearance.
Vaginal pH is almost always greater than 4.5. A positive whiff test is not unusual. The vaginal wet preparation contains an abundance of leukocytes and motile flagellated trichomonads (Fig. 110-6). In experienced hands, the wet preparation is only 60% to 70% sensitive in symptomatic patients.15 The organism can be cultivated by inoculating a liquid medium such as modified Diamond’s medium14 or InPouch TV.16 After incubation, aliquots of the liquid medium are examined daily for motile trichomonads. Culture improves the diagnostic yield, especially in asymptomatic patients. Nonculture tests approved by the U.S. Food and Drug Administration (FDA) include OSOM Trichomonas Rapid Test (Genzyme, Cambridge, MA), Affirm VPIII (Becton Dickinson, Franklin Lakes, NJ), and XenoStrip-Tv (Xenotope Diagnostics, San Antonio, TX). These tests have sensitivity that approaches that of culture.16,17 Sensitive and specific nucleic acid amplification tests have also been developed (including APTIMA Trichomonas vaginalis Assay [Gen-Probe, San Diego, CA])18,19
A wet preparation diagnostic of trichomoniasis is highly specific because of the characteristic motility of the organisms. Diagnostic tests in which the organisms are no longer motile may lack specificity. A common clinical problem is the woman with no epidemiologic evidence for a sexually transmitted condition who has trichomonads visualized on a cervical cytologic examination.20 In some of these cases the cytologist may have misread the smear. For such a patient, the clinician should obtain confirmation of the diagnosis of trichomoniasis, by wet preparation or culture (or both), before initiating treatment and before embarking on a potentially disruptive epidemiologic investigation.
Therapy
Metronidazole and tinidazole are the only effective agents that are approved by the FDA for the treatment of trichomoniasis. A single 2-g oral dose of metronidazole or tinidazole can be prescribed. Alternatively, 500 mg of oral metronidazole can be given twice daily for 7 days. Controlled studies have failed to show any important advantage of the 7-day regimen. A single-dose regimen, if administered under direct observation in the office or clinic, has the obvious advantage of 100% compliance. Because trichomoniasis is almost always sexually transmitted, treatment with metronidazole of all recent sexual partners, regardless of their symptoms, is an integral part of management.21
The aforementioned regimens containing metronidazole or tinidazole eliminate trichomonads in well over 90% of instances. If this treatment fails (Table 110-8), the diagnosis should be reconfirmed with a wet preparation or culture. Additionally, treatment of all current sexual partners should be ensured. Initial re-treatment should be with oral metronidazole, 500 mg twice daily for 7 days, or a single 2.0-g dose of oral tinidazole. If this regimen is not successful, oral metronidazole or tinidazole in a single daily dose of 2.0 g can be prescribed for 5 days.21 If the latter metronidazole regimen fails, the patient can be assumed to have clinically significant metronidazole resistance, and consultation with an expert in the management of trichomoniasis should be sought. Susceptibility testing, available through the Centers for Disease Control and Prevention, should be performed.22 Alternative regimens include very high doses of metronidazole. One such regimen is that described by Lossick and associates,23 which consists of both oral (2.5 g daily) and vaginal (0.5 g daily) metronidazole for up to 3 weeks. Antiemetics may be prescribed with this regimen to reduce nausea and vomiting. In Lossick’s experience, this regimen was effective in about 90% of cases. Intravenous regimens of metronidazole have also been prescribed.24 For patients who cannot tolerate metronidazole at this dosage level and for those who do not respond to it, tinidazole has better in vitro efficacy,25 is better tolerated than metronidazole, and has cured most patients with metronidazole-resistant trichomoniasis.26
For patients with trichomoniasis who are unresponsive to metronidazole and tinidazole, there are few therapeutic options of proven value. Nonoxynol-9 is active against T. vaginalis in vitro and was reported to be effective in one patient.27 However, in a subsequent study, nonoxynol-9 was effective in only 3 of 17 patients.28 Furazolidone is highly active in vitro against metronidazole-sensitive and metronidazole-resistant isolates of T. vaginalis29 and was effective when given vaginally in a study conducted some years ago.30 In one study,31 vaginal acidification with boric acid was effective. In general, however, topical treatments have been disappointing,14 presumably because of reservoirs of infection in periurethral glands and other areas that are not adequately sterilized by intravaginal medications. Specifically, topical preparations containing metronidazole (e.g., 0.75% metronidazole gel) have been ineffective in unselected patients with trichomoniasis32 and are of no use in patients with metronidazole resistance except as a possible adjunct to oral treatment.
T. vaginalis organisms have been shown to be estrogen dependent in vitro33 and in vivo.34 In one report, discontinuation of estrogen replacement treatment in a postmenopausal woman was associated with resolution of vaginal trichomoniasis.35 These data suggest that hormonal manipulation should be studied in the management of trichomoniasis that is unresponsive to metronidazole and tinidazole and for women who cannot tolerate these drugs.
Minor adverse reactions to metronidazole, primarily nausea and a metallic taste, are common, but most patients can tolerate the usual dosage schedules. Metronidazole allergy is unusual. In such instances, desensitization has been useful.36
HIV infection has no effect on the incidence or prevalence of trichomoniasis or on persistence or recurrence.37 Treatment of trichomoniasis decreases the viral load of HIV in vaginal fluid,38 an observation concordant with the results of studies that show an association of trichomoniasis with HIV acquisition.39
Trichomoniasis in pregnancy has been the subject of considerable interest. An association between trichomoniasis and premature rupture of the membranes was reported in 1984.40 Cotch and colleagues,41 using data from the Vaginal Infections and Prematurity (VIP) study, reported in 1997 on the prospective evaluation of 13,816 pregnant women. They found that trichomoniasis was independently associated with a 30% greater likelihood of preterm delivery and low birth weight and a 40% greater likelihood of having a preterm infant of low birth weight. In a more recent study, treatment of trichomoniasis in asymptomatic pregnant women with metronidazole did not prevent preterm delivery.42 Metronidazole has traditionally been avoided during pregnancy because of largely theoretical concerns about mutagenicity and oncogenicity. However, studies and meta-analyses have not demonstrated a consistent association between metronidazole use during pregnancy and teratogenic or mutagenic effects in infants.43–45 Therefore, pregnant women who have symptomatic trichomoniasis may be treated with 2 g of metronidazole.21 Tinidazole has not been well evaluated in pregnant women. In lactating women, breast-feeding should be stopped during treatment and for 12 to 24 hours after completion of treatment with metronidazole and for 3 days after completion of treatment with tinidazole.21
Vulvovaginal Candidiasis
Etiology and Pathogenesis
Candida albicans and other species of Candida can be part of the vaginal microbiota of asymptomatic women. About 30% of unselected women are colonized. In one study of unselected women,13 two thirds of colonized women and only 22% of uncolonized women reported symptoms, primarily vulvovaginal itching and irritation. These data suggest that colonization with Candida species usually produces symptoms, albeit mild symptoms that do not prompt the patient to seek medical attention.
C. albicans can be isolated from 80% to 90% of patients with vulvovaginal candidiasis, and other yeasts account for up to 20% of cases.46 Candida tropicalis is isolated from 1% to 5% and may be associated with a higher rate of recurrence after standard treatments.47,48 Candida (formerly Torulopsis) glabrata accounts for about 10% of vaginal yeast isolates.46,47,49–51 Symptomatic vaginitis caused by this organism is associated with less intense itching and dyspareunia49 than that caused by other Candida species, but the organism may be harder to eradicate with standard therapies.47,51 The relative incidence of vaginitis caused by fungi other than C. albicans appears to be increasing. Non-albicans infections are associated with recurrent disease (accounting for 21% of recurrent vs. 12% of initial infections) and with HIV infection (22% of infections in HIV-positive women vs. 12% in HIV-negative women), especially in HIV-infected women who receive prophylaxis with imidazoles or triazoles.47 It is believed that the widespread use of topical antifungal agents, especially in short courses, may contribute to selection for non-albicans yeasts, which are less susceptible to these agents than is C. albicans. Cases of vaginitis caused by Saccharomyces cerevisiae have been reported and may be associated with baking.52,53
Some workers have estimated that 75% of adult women will suffer at least one episode of vulvovaginal candidiasis during their lifetime.54 Inhibition of normal bacterial microbiota by antibiotics favors the growth of yeasts,54,55 although symptomatic cases are seen after the use of antimicrobial agents that do not suppress lactobacilli.56 Vulvovaginal candidiasis sometimes occurs after antimicrobial treatment of trichomoniasis or BV.
Growth of yeasts is apparently favored by high estrogen levels, although such levels also promote the growth of lactobacilli.57–59 The prevalence of vaginal carriage of Candida is higher among users of oral contraceptives than among women using other methods of birth control.55,59 The mechanism of this estrogenic predisposition is unclear.
Vulvovaginal candidiasis is associated with poorly controlled diabetes mellitus, and tight glycemic control decreases the frequency of symptomatic infection.60 However, testing for diabetes in women with recurrent vulvovaginal candidiasis is not cost effective.55
It has been suggested that tight, insulating clothing predisposes to vulvovaginal candidiasis by increasing vulvar warmth and moisture. In prospective studies, a higher prevalence of candidal carriage and higher concentrations of organisms were found in women who wore tight rather than loose clothing.61–63 Impairment of phagocytic cells or of cell-mediated immunity (e.g., transplantation, chemotherapy) also predisposes to vulvovaginal candidiasis. Some authorities believe that women with HIV infection develop vulvovaginal candidiasis more often than HIV-negative women do, especially if they have low CD4 T-cell counts.64,65
The contribution of sexual transmission is poorly defined. Vulvovaginal candidiasis increases in incidence with the onset of sexual activity,66–68 but the incidence is also increased by the use of oral contraceptives,55,59 the contraceptive sponge, or the intrauterine device,67 any of which might coincide with sexual activity. Having multiple sexual partners is not associated with a higher incidence of Candida infection. Most women who present with vulvovaginal candidiasis have no predisposing illnesses or medications.
The mechanism by which Candida produces disease is not well defined. Although it is postulated that differences in virulence must exist,55 strains isolated from symptomatic women are not demonstrably different from isolates from asymptomatic carriers.69 Filamentous forms (hyphae and pseudohyphae) are associated with active disease.70 Pseudohyphae have been observed to penetrate vaginal epithelial cells,71 and they are more adherent to cells than are budding yeasts (blastospores).72 Adherence appears to be an important pathogenic feature of Candida spp.,73 and sublethal concentrations of antifungal agents may ameliorate disease by reducing adherence.74
The severity of symptoms in vulvovaginal candidiasis is not directly related to the number of yeast cells present. Indeed, very small numbers of yeasts may be present in vaginal material recovered from highly symptomatic women.55 An immunologic reaction has been suggested as the mechanism for symptomatic disease in such women,75 and one small series suggested that desensitization may decrease the frequency of symptomatic episodes.76
Clinical Manifestations
Patients with candidal vulvovaginitis generally complain of perivaginal pruritus, often with little or no discharge (Table 110-9). Dysuria is occasionally noted and is likely to be perceived as vulvar rather than urethral. The labia may be pale or erythematous. Shallow, radial, linear ulcerations (Fig. 110-7), especially on the posterior portion of the introitus, are common. Excoriations caused by scratching are often present (Fig. 110-8). Tiny papules or papulopustules, called satellite lesions, just beyond the main area of erythema are helpful diagnostically. The vaginal walls may be erythematous. Candidal discharge is classically thick and adherent (Fig. 110-9). However, it may be thin and loose, resembling the discharge of other vaginitides.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree