Jerod L. Nagel, David M. Aronoff
Metronidazole
Metronidazole (1-[2-hydroxyethyl]-2-methyl-5-nitroimidazole) is a nitroimidazole drug active against anaerobic bacteria and certain parasites. It is a widely used drug given its tolerability, high oral bioavailability, and capacity to penetrate into tissues well, including the central nervous system.
History
Metronidazole was first synthesized in the 1950s when the pharmaceutical company Rhône-Poulenc was searching for an effective antitrichomonal drug for the treatment of vaginal trichomoniasis.1 Initially, a crude extract of a Streptomyces bacterium was found to kill Trichomonas vaginalis and the active component was determined to be azomycin, a previously characterized nitroimidazole antibiotic.2 Metronidazole was a synthetic analogue of azomycin that was both more active against T. vaginalis and less toxic.1,3 It was initially called 8823 R.P.1 This discovery soon led to its successful use in human clinical studies of trichomoniasis.4,5 The presumed antibacterial activity of metronidazole was discovered incidentally by Shinn in 1962, who reported improvement in ulcerative gingivitis in a patient treated for a concomitant T. vaginalis infection, a result he confirmed in a series of patients with ulcerative stomatitis.6
Mechanism of Action
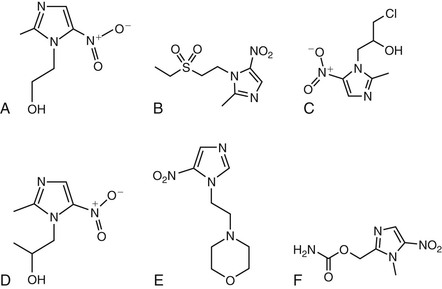
Metronidazole and other nitroimidazoles (e.g., nimorazole, ornidazole, ronidazole, secnidazole, tinidazole) are inert, and their spectrum of antimicrobial activity is determined by the capacity of susceptible organisms to activate the drugs once they enter the cell via passive diffusion. The structures of metronidazole and related compounds are illustrated in Figure 28-1. Their bactericidal and parasiticidal activities are rapid and proportional to the concentration of the activated drugs within the target cell.7 The members of this class of antibiotics are therefore best considered as prodrugs, which are activated through a reduction step to form highly reactive products that interact with intracellular targets (see later).8 Metronidazole’s mechanism of action describes that of the other nitroimidazoles.
The nitroimidazoles share a heterocyclic structure consisting of an imidazole-based nucleus with a nitro group, NO2, in position 5. There are four major steps involved in the mechanism of action of metronidazole that result in the intracellular formation of critical redox intermediate metabolites.9 In the first two steps, the drug enters cells by passive diffusion and an electron is transferred to the nitro group of metronidazole, resulting in the production of a short-lived nitroso free radical, which is cytotoxic and can interact with cellular DNA.10 This process of activating the prodrug creates a concentration gradient that augments the increased uptake of the drug by the organism, further increasing its antimicrobial effect. The third step in metronidazole’s action relates to the cytotoxic effect of the reduced product because the activated metronidazole compound can inhibit DNA synthesis and induce DNA damage via oxidation, resulting in single-strand and double-strand breaks.10 Thus, metronidazole induces DNA degradation and cell death.10 Finally, there is the release of inactive end products of the drug.11
The microbial selectivity of metronidazole reflects the inability of aerobic bacteria to activate the prodrug because they lack the necessary electron transport proteins with sufficient negative redox potential.10 However, in susceptible anaerobic bacteria, the redox potential of components of the electron transport chain is sufficiently negative to reduce the nitro group of metronidazole. The drug is activated in anaerobic bacteria when it receives an electron from ferredoxin or flavodoxin, which are themselves reduced by iron-sulfur proteins called pyruvate:ferredoxin oxidoreductases (PFORs).12 The exact electron donors involved in nitroimidazole reduction vary, depending on the organism.10 In the microaerophile Helicobacter pylori, for example, a separate mechanism appears to be involved in metronidazole susceptibility, involving a 2-electron transfer step mediated by an oxygen-insensitive nitroreductase (RdxA).10 Several microaerophilic protists (Giardia lamblia, Entamoeba histolytica, and T. vaginalis) have bacteria-like enzymes (nitroreductases) capable of activating metronidazole.13
Spectrum of Activity
Metronidazole and related nitroimidazoles are active against a variety of anaerobic bacteria, as well as microaerophilic bacteria and protozoa. Resistance, as detailed later, has been increasingly detected in certain organisms, though this may not be identified easily because sensitivity testing for anaerobes is not performed routinely. However, the emergence of resistance suggests that ongoing surveillance is important.10
Many gram-negative anaerobes are susceptible to metronidazole.14,15 As a rule, members of the genera Bacteroides and Parabacteroides are susceptible to metronidazole, with resistance generally detected in less than 5% of isolates.16–19 Higher rates of resistance have been reported among Bacteroides isolates in South Africa.20 Desulfovibrio species are also highly susceptible,21 and clinically relevant members of the Fusobacterium, Porphyromonas, Prevotella, and Bilophila genera are usually sensitive.14,22 However, nonsusceptible Prevotella strains have been described.14 Recently, metronidazole resistance has been reported in oral isolates of Porphyromonas gingivalis.23 Reduced susceptibility has also been noted in isolates of Sutterella14 and in the gram-negative coccus Veillonella.15,24 The bacterial vaginitis-associated bacteria of the genus Mobiluncus are usually not susceptible to metronidazole.25,26
Facultative anaerobes have variable susceptibility to metronidazole and are generally not empirically treated with this agent. Aggregatibacter actinomycetemcomitans is occasionally susceptible, but resistance is sufficiently common that empiric metronidazole should not be used for these infections.23,27 Another oral, facultative anaerobe, Eikenella corrodens, is generally resistant to the nitroimidazoles.28 The CO2-requiring members of the genus Capnocytophaga are generally resistant to metronidazole.29 Although Campylobacter jejuni and Campylobacter coli isolates may be susceptible in vitro to metronidazole, the drug is not recommended for therapy against these pathogens.30 Gardnerella vaginalis, which is associated with bacterial vaginosis, is variable in its sensitivity, with nearly 30% of isolates in one series demonstrating resistance, suggesting that resistance should be considered in cases of treatment failure.31 A more recent survey from India documented nearly 70% of G. vaginalis strains were metronidazole resistant, but the cases included metronidazole-exposed women exhibiting recurrent infection.32 The clinical relevance of in vitro resistance of Gardnerella to metronidazole is difficult to interpret because a hydroxy metabolite of metronidazole is actually more active against G. vaginalis than the parent compound.33,34
H. pylori is a facultative anaerobe that was initially sensitive to metronidazole but has increasingly developed clinically important resistance. A recent survey of 10,670 clinical isolates obtained from H. pylori–treated and H. pylori–untreated individuals revealed metronidazole resistance in 17.4% of isolates from previously untreated children and 26.1% of strains from previously untreated adults. Resistance rates in strains obtained from antibiotic-exposed children and adults were higher, at 37.7% and 49.0%, respectively.35 Treatment failure for H. pylori was a significant risk factor for harboring a metronidazole-resistant isolate.35
Among the gram-positive anaerobes, the clostridia remain quite susceptible to metronidazole.36 However, reduced susceptibility to metronidazole has been noted among a limited number of Clostridium difficile isolates.37–39 Notably, the Etest overestimates metronidazole susceptibility in C. difficile isolates, compared with the more involved agar-incorporation-based methods for determining minimal inhibitory concentrations (MICs).37,39 Whether the clinical response to metronidazole is affected by reduced in vitro susceptibility remains to be determined. However, this deserves closer attention in light of the highly variable concentrations of metronidazole in the stools of treated patients40,41 and increasing reports of treatment failure and recurrence of C. difficile infection (CDI) in metronidazole-treated patients.42
An important hole in the anaerobic spectrum of metronidazole is found in its lack of activity against a number of non–spore-forming, gram-positive anaerobic bacteria that possess intrinsic resistance to the drug. These include isolates of Actinomyces, Bifidobacterium, Lactobacillus, and Propionibacterium.10,36,43 Propionibacterium acnes is highly resistant.15,36,44 Metronidazole should not be routinely used to treat infections with these organisms unless susceptibility is confirmed. In contrast, the genus Eubacterium is generally sensitive to metronidazole in vitro.36
The nitroimidazoles possess good activity against several protozoa. Apart from T. vaginalis, metronidazole has activity against Giardia (syn. G. duodenalis, G. lamblia, G. intestinalis) and Entamoeba histolytica. Resistance is uncommon in Giardia, and clinical efficacy is generally greater than 90%, but in vitro testing has revealed reduced susceptibility to metronidazole in clinical isolates, causing concern.45,46 Nitroimidazoles exhibit in vitro activity against Dientamoeba fragilis, which is a trichomonad known to cause gastroenteritis.47
Apart from its antimicrobial actions, metronidazole exhibits immunosuppressive and anti-inflammatory actions48 and has been used effectively in the treatment of rosacea,49 although the extent to which this relates to metronidazole’s antibacterial properties is unclear.
Effects on the Human Microbiome
Metronidazole, like many antimicrobials, affects the human microbiome, although because this has mostly been examined in subjects exposed to metronidazole in combination with other antimicrobials, it is difficult to fully understand the impact of metronidazole itself.10,50 Early culture-based studies of the impact of metronidazole monotherapy on bacterial communities in the human gastrointestinal tract described little impact of the drug on microbial populations.41,51 However, some investigators reported a suppression of anaerobes and a relative increase in the abundance of certain aerobic bacteria (Escherichia coli and fecal streptococci).52 The reasons for the relatively low impact on normal gut microbes are not well understood but may relate to the pharmacology of metronidazole, which achieves low concentrations in the feces of healthy adults.40,53 At present there are few studies of metronidazole’s impact on the gastrointestinal microbiome that have used culture-independent techniques such as DNA pyrosequencing, apart from studies involving antibiotic combinations.50 This is an area in need of new research.
Nucleic acid sequencing methods have been applied to understand the impact of metronidazole on the microbiome of the female reproductive tract, particularly in the context of bacterial vaginosis. Topical metronidazole has recently been evaluated for its impact on the vaginal microbiome in women with bacterial vaginosis.54–56 Five to seven days of metronidazole consistently reduced the diversity of bacterial communities in these studies of bacterial vaginosis, compared with no treatment, and for many women it restored a more normal, Lactobacillus-dominated mucosal microbiome.54–56
Pharmacology
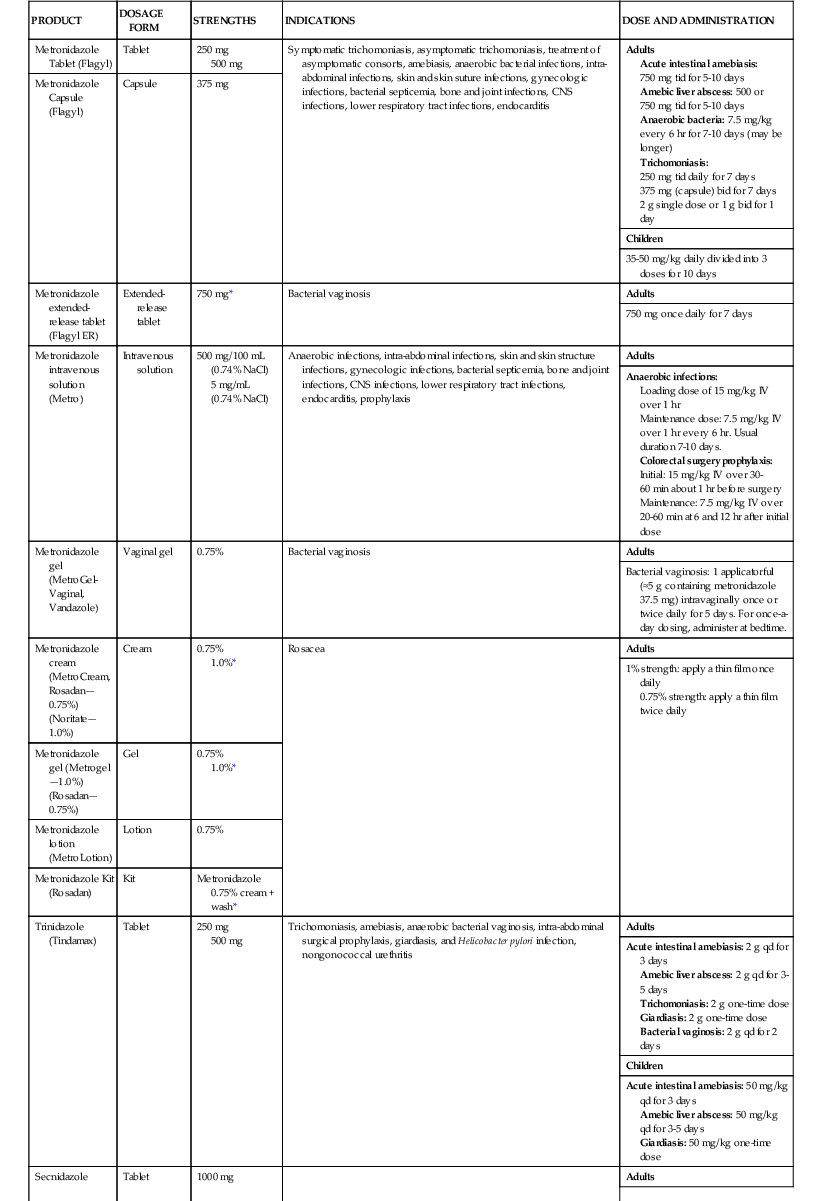
Metronidazole is commercially available in a variety of formulations: oral capsules and tablets (immediate and extended release); intravenous solution; topical gels, creams, and lotions; and vaginal gels.7,57 Although oral metronidazole suspension is not commercially available, it is commonly compounded in pharmacies by crushing immediate release tablets and mixing with a 1 : 1 ratio of an aqueous suspending solution and buffered oral syrup.58 The dose and duration of metronidazole is dependent on the specific product and indication (Table 28-1). An IV loading dose of 15 mg/kg, followed by 7.5 mg/kg every 6 to 8 hours, is recommended in the package insert, with a maximum daily dose limit of 4 g.7 A fixed dose of 500 mg IV every 8 hours maintains concentrations above typical MICs for Bacteroides species and is effective for treatment of intra-abdominal infections.59–61 An infusion time of 1 hour is traditionally recommended, but 20- to 30-minute infusions have been used.62 Given the long half-life and concentration-dependent activity, high-dose metronidazole, administered as 1 to 1.5 g every 24 hours, may be a safe and effective alternative to 500 mg every 6 to 8 hours.63,64 The typical duration of oral or intravenous metronidazole courses ranges from 1 to 10 days depending on the indication and patient condition. Longer durations may be prescribed, but caution should be exercised with durations greater than 1 month due to increased risk of peripheral neuropathy and central nervous system adverse effects.65–67
TABLE 28-1
Major Preparations and Indications for Metronidazole: Administration and Dosage
| PRODUCT | DOSAGE FORM | STRENGTHS | INDICATIONS | DOSE AND ADMINISTRATION |
| Metronidazole Tablet (Flagyl) | Tablet | 250 mg 500 mg | Symptomatic trichomoniasis, asymptomatic trichomoniasis, treatment of asymptomatic consorts, amebiasis, anaerobic bacterial infections, intra-abdominal infections, skin and skin suture infections, gynecologic infections, bacterial septicemia, bone and joint infections, CNS infections, lower respiratory tract infections, endocarditis | Adults Acute intestinal amebiasis: 750 mg tid for 5-10 days Amebic liver abscess: 500 or 750 mg tid for 5-10 days Anaerobic bacteria: 7.5 mg/kg every 6 hr for 7-10 days (may be longer) Trichomoniasis: 250 mg tid daily for 7 days 375 mg (capsule) bid for 7 days 2 g single dose or 1 g bid for 1 day |
| Metronidazole Capsule (Flagyl) | Capsule | 375 mg | ||
| Children | ||||
| 35-50 mg/kg daily divided into 3 doses for 10 days | ||||
| Metronidazole extended-release tablet (Flagyl ER) | Extended-release tablet | 750 mg* | Bacterial vaginosis | Adults |
| 750 mg once daily for 7 days | ||||
| Metronidazole intravenous solution (Metro) | Intravenous solution | 500 mg/100 mL (0.74% NaCl) 5 mg/mL (0.74% NaCl) | Anaerobic infections, intra-abdominal infections, skin and skin structure infections, gynecologic infections, bacterial septicemia, bone and joint infections, CNS infections, lower respiratory tract infections, endocarditis, prophylaxis | Adults |
| Anaerobic infections: Loading dose of 15 mg/kg IV over 1 hr Maintenance dose: 7.5 mg/kg IV over 1 hr every 6 hr. Usual duration 7-10 days. Colorectal surgery prophylaxis: Initial: 15 mg/kg IV over 30-60 min about 1 hr before surgery Maintenance: 7.5 mg/kg IV over 20-60 min at 6 and 12 hr after initial dose | ||||
| Metronidazole gel (MetroGel-Vaginal, Vandazole) | Vaginal gel | 0.75% | Bacterial vaginosis | Adults |
| Bacterial vaginosis: 1 applicatorful (≈5 g containing metronidazole 37.5 mg) intravaginally once or twice daily for 5 days. For once-a-day dosing, administer at bedtime. | ||||
| Metronidazole cream (MetroCream, Rosadan—0.75%) (Noritate—1.0%) | Cream | 0.75% 1.0%* | Rosacea | Adults |
| 1% strength: apply a thin film once daily 0.75% strength: apply a thin film twice daily | ||||
| Metronidazole gel (Metrogel—1.0%) (Rosadan—0.75%) | Gel | 0.75% 1.0%* | ||
| Metronidazole lotion (MetroLotion) | Lotion | 0.75% | ||
| Metronidazole Kit (Rosadan) | Kit | Metronidazole 0.75% cream + wash* | ||
| Trinidazole (Tindamax) | Tablet | 250 mg 500 mg | Trichomoniasis, amebiasis, anaerobic bacterial vaginosis, intra-abdominal surgical prophylaxis, giardiasis, and Helicobacter pylori infection, nongonococcal urethritis | Adults |
| Acute intestinal amebiasis: 2 g qd for 3 days Amebic liver abscess: 2 g qd for 3-5 days Trichomoniasis: 2 g one-time dose Giardiasis: 2 g one-time dose Bacterial vaginosis: 2 g qd for 2 days | ||||
| Children | ||||
| Acute intestinal amebiasis: 50 mg/kg qd for 3 days Amebic liver abscess: 50 mg/kg qd for 3-5 days Giardiasis: 50 mg/kg one-time dose | ||||
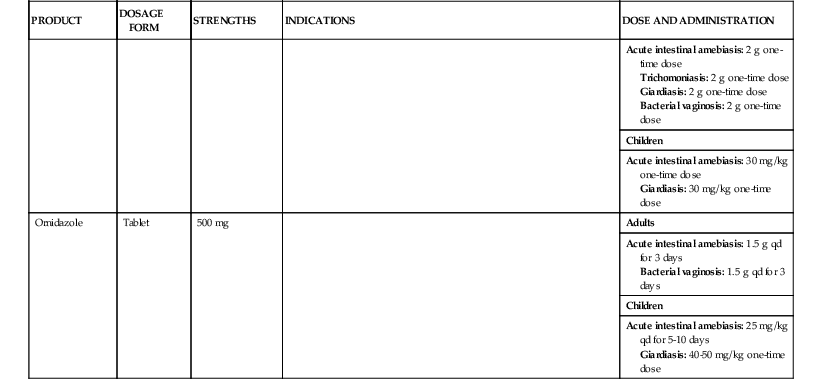
| Secnidazole | Tablet | 1000 mg | Adults | |
| Acute intestinal amebiasis: 2 g one-time dose Trichomoniasis: 2 g one-time dose Giardiasis: 2 g one-time dose Bacterial vaginosis: 2 g one-time dose | ||||
| Children | ||||
| Acute intestinal amebiasis: 30 mg/kg one-time dose Giardiasis: 30 mg/kg one-time dose | ||||
| Ornidazole | Tablet | 500 mg | Adults | |
| Acute intestinal amebiasis: 1.5 g qd for 3 days Bacterial vaginosis: 1.5 g qd for 3 days | ||||
| Children | ||||
| Acute intestinal amebiasis: 25 mg/kg qd for 5-10 days Giardiasis: 40-50 mg/kg one-time dose |
* No generic available.
CNS, central nervous system.
Oral metronidazole is rapidly and almost completely absorbed, with bioavailability approaching 100%.7,57 When rectally administered, metronidazole is also well absorbed with reported bioavailability of 59% to 94%; topical and vaginal metronidazole achieves detectable systemic concentrations with bioavailability ranging from 2% to 25%.7,57,68 Administration of oral metronidazole with food is encouraged to minimize gastrointestinal adverse effects and does not affect bioavailability but may delay the time to peak serum concentrations. Peak serum concentrations range from 12 to 40 µg/mL and occur 1 to 2 hours after oral administration and approximately 3 hours after rectal administration.7,57
Metronidazole is a lipophilic molecule with low protein binding and moderate to large volume of distribution, allowing extensive distribution into various tissues (Table 28-2).7,57 Penetration into inflamed cerebrospinal fluid, epithelial lining fluid, saliva, and bile is excellent and concentrations are similar to serum.7,57,69 Patients with noninflamed meninges still achieve therapeutic concentrations at approximately 43% of serum.70 Additionally, penetration into abscesses, appendix tissue, peritoneal fluid, and pancreatic tissue is very good, ranging from 2.3 to 7.2 µg/mL.7,57,71 However, patients with obstructive cholecystitis have negligible amounts of drug detected in the bile.7,57 Metronidazole crosses the placental barrier and penetrates into breast milk and may be teratogenic during the first trimester (see “Precautions”).72 Stool concentrations during C. difficile colitis are highest at the beginning of infection and taper as inflammation subsides and stool is formed, but concentrations generally remain well above reported MICs.40 This effect of higher stool concentrations when diarrhea is present is also noted during flares of Crohn’s disease.41
TABLE 28-2
Pharmacokinetic and Pharmacologic Properties of Metronidazole
| Pharmacologic or Pharmacokinetic Factor | Result | Comments |
| Absorption | ||
| Oral | 98%-100% | |
| Rectal | 59%-94% | |
| Vaginal cream | 20% | |
| Vaginal gel | 56% | |
| Topical | 2% | |
| Time to Peak | ||
| Oral | 1-2 hr | |
| Rectal | 3 hr | |
| Topical | 8-12 hr | |
| Peak Serum Concentrations | ||
| Intravenous | 25 and 18 µg/mL | After 15 mg/kg load and 7.5 mg/kg every 6 hr |
| Oral | 6, 12, 21.4, and 40 µg/mL | After single dose of 250 mg, 500 mg, 750 mg, and 2000 mg |
| Rectal | 18.5 µg/mL | After 500-mg dose |
| Topical | 27.5 µg/mL | After application of 1% cream |
| Volume of Distribution | ||
| Adults | 0.55 L/kg | |
| Neonates | 0.54-0.81 L/kg | |
| Tissue and Fluid Penetration | ||
| CSF (inflamed meninges) | Approximates serum concentration | |
| CSF (noninflamed meninges) | 45% of serum concentration | |
| Bile | Approximates serum concentration | |
| Epithelial lining fluid | Approximates serum concentration | |
| Saliva | Approximates serum concentration | |
| Abscess | Variable, but high concentration | |
| Peritoneal fluid | High concentrations: 7.2-14.2 µg/mL | |
| Pancreatic tissue | High concentration: 5.1-8.5 µg/mL | |
| Metabolism | ||
| Oxidation | Primary mechanism of elimination | |
| Glucuronidation | Secondary mechanism of elimination | |
| Cytochrome P450 | Secondary mechanism of elimination | |
| Excretion | ||
| Unchanged drug | 6%-18% | |
| Metabolites | 60%-80% | |
| Hemodialysis | Removes 25%-45% over 4 hr | |
| Peritoneal dialysis | Removes 10% over 7.5 hr | |
| Protein Binding | <20% | |
| Pregnancy | Avoid in first trimester Category B | |
| Lactation | Avoid | Significant penetration into breast milk |
CSF, cerebrospinal fluid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree