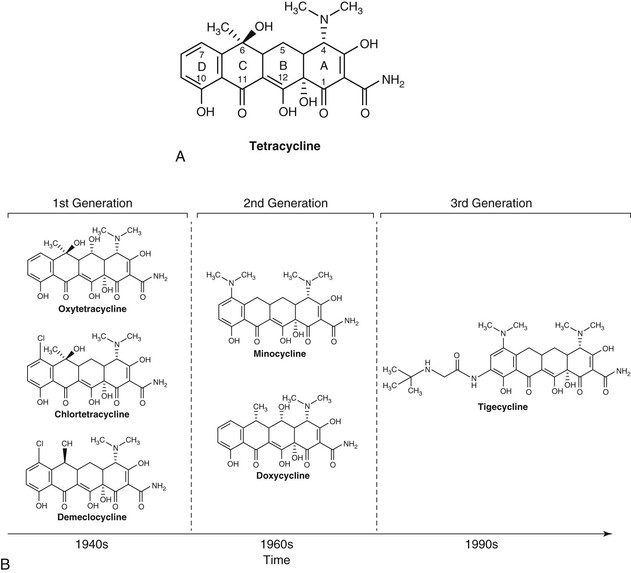
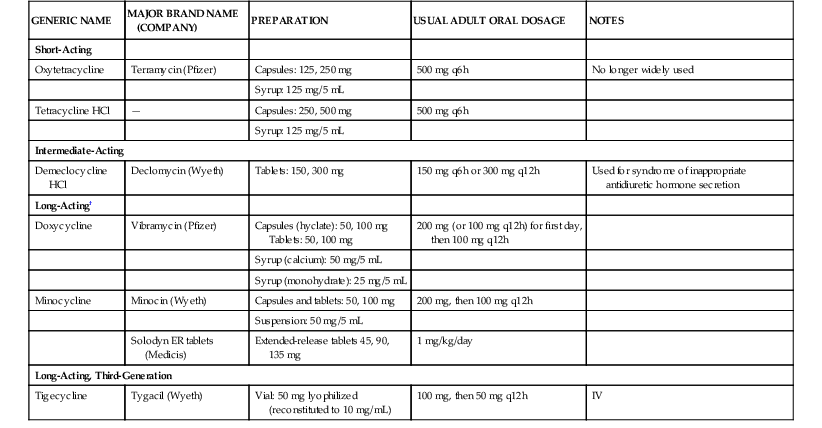
Matthew Moffa, Itzhak Brook Tetracyclines have been an important class of broad-spectrum antibiotics since the discovery of chlortetracycline in 1948 by mycologist Benjamin M. Duggar.1 They are bacteriostatic with a wide range of activity, including gram-positive bacteria, gram-negative bacteria, intracellular organisms, and protozoan parasites. Duggar derived chlortetracycline from Streptomyces aureofaciens, a golden yellow bacterium found in soil. In 1950, oxytetracycline was isolated from Streptomyces rimosus. Tetracycline was later prepared by the catalytic dehalogenation of chlortetracycline in 1953 at Lederle Laboratories, as well as being independently derived from oxytetracycline at Pfizer Laboratories during that same time period.2,3 Doxycycline is a semisynthetic derivative of oxytetracycline and became available in 1967. Minocycline, also derived semisynthetically, was derived in 1972. Shortly after the discovery of tetracyclines, resistance developed due in large part to their extensive clinical and nonclinical uses, including growth promoters in animal feeds.4,5 This widespread usage has selected for a large number of resistant determinants collectively termed the tetracycline resistome.6 This resistance led to a period of time when tetracyclines were replaced by newer antibiotics, such as the fluoroquinolones in the 1970s and 1980s. Like every other antibiotic, the fluoroquinolones would also fall to the selective pressure of antimicrobial resistance, leading to the development of newer, semisynthetic tetracyclines called glycylcyclines. Tigecycline was the first glycylcycline approved by the U.S. Food and Drug Administration (FDA) in 2005 for treatment of complicated skin and soft tissue infections, as well as complicated intraabdominal infections. Later, it was approved for community-acquired pneumonia in 2009. Tetracyclines, as a class, are commonly divided into two distinct classification methods, by either duration of action or year of discovery (Table 26-1, Fig. 26-1). Short-acting tetracyclines include the first-generation oxytetracycline and tetracycline. Intermediate-acting tetracyclines include another first-generation member, demeclocycline. Of note, demeclocycline is rarely used for infections. Its main side effect is nephrogenic diabetes insipidus. Thus, it has found its niche treating hyponatremia in the setting of the syndrome of inappropriate antidiuretic hormone secretion, first reported in the 1970s.7 Long-acting tetracyclines include the second-generation doxycycline and minocycline, as well as the third-generation glycylcycline, tigecycline. TABLE 26-1 Tetracycline Formulations Currently Available in the United States* * Other brands are available for some of the analogs. † Long-acting agents exist in intravenous preparations and can be given at the same doses recommended for oral therapy (doxycycline 100-mg vial, 200-mg vial; minocycline, 100-mg vial). Tetracyclines all share a four benzene ring as their core structure, with a hydronaphthacene nucleus (see Fig. 26-1). Variations in gastrointestinal absorption, affinity for multivalent cations, protein binding, and antimicrobial activity can be achieved with substitutions at carbons 5, 6, and 7 of the four-ring, six-membered carbocyclic structure, leading to variations in pharmacokinetic properties.3 Tetracyclines work by inhibiting bacterial protein synthesis. This is accomplished primarily by reversibly binding to the 30S ribosomal subunit of the bacteria. This inhibits the enzyme binding of aminoacyl-tRNA to the adjacent ribosomal acceptor site, which in turn prevents peptide chain elongation and inhibits protein synthesis.2 Because the binding of tetracyclines to the 30S ribosomal subunit is reversible, it is postulated that this is the explanation as to why they exhibit bacteriostatic properties. In order for tetracyclines to get to the 30S ribosomal subunit, they need to be able to penetrate cell walls, which is accomplished by passive diffusion. With gram-negative organisms, tetracyclines become positively charged cation complexes, presumably with magnesium. They then use OmpF and OmpC porin channels to cross the outer membrane. After entering the periplasmic space, tetracycline dissociates, resulting in an accumulation of uncharged tetracycline. With gram-positive organisms, tetracyclines penetrate through the inner cytoplasmic membrane via an active transport system that depends on the ΔpH.3,8 Tetracyclines also reduce bacterial pathogenicity by suppressing adhesion of bacteria to human cells. This is likely achieved by inhibiting the synthesis of a specific protein in the bacterial cell surface.9 Doxycycline displays additional protein synthesis inhibition in mitochondria through binding of the 70S ribosomes.10 This enables it to exhibit activity against various protozoa because they contain mitochondria. Doxycycline also targets parasites via the apicoplast ribosomal subunits in Plasmodium falciparum. This occurs late in the malarial cell cycle, resulting in the slow antimalarial effect of doxycycline.10,11 Tetracycline HCl comes in 250 mg and 500 mg capsules, as well as 125 mg/5 mL syrup. The usual adult oral dosage is 250 mg every 6 hours or 500 mg every 6 hours for more serious infections. Larger doses do not provide additional benefit and the excess drug is excreted in feces. Intravenous preparations of tetracycline are no longer used due to their potential hepatotoxicity. Tetracycline is labeled category D and is to be avoided in pregnancy. It should also be avoided in children, particularly those younger than 8 years old during the period of tooth development to avoid permanent discoloration. Children are also at risk for bone developmental retardation. With renal impairment, tetracycline should be avoided because it can cause further deterioration of kidney function and the drug tends to accumulate in the serum.12 Tetracycline is slowly removed by hemodialysis, but not very well by peritoneal dialysis.13 Caution is advised in administering tetracycline in patients with hepatic impairment because this may result in liver damage. Doxycycline comes in various doses, but most typically in both 50 mg and 100 mg capsules and tablets. It is also available as 25 mg/5 mL and 50 mg/5 mL syrup. The usual adult dosage is 100 mg every 12 hours and should be taken with at least 100 mL of water. For malaria chemoprophylaxis, the adult dosage is 100 mg daily. Occasionally an intravenous preparation of doxycycline is required for certain cases of rickettsial infections, ehrlichiosis, or severe psittacosis. The usual adult dosage is 200 mg followed by 100 mg every 12 hours. The dose should be given over 30 to 60 minutes and be dissolved in 500 to 1000 mL of glucose or saline.10 Like tetracycline, doxycycline is also pregnancy category D and should be avoided in children. If the benefits of doxycycline outweigh the risks in children, the pediatric dose is 2.2 mg/kg every 12 hours. Unlike tetracycline, doxycycline is safe to use with renal impairment. Urinary excretion is reduced in renal impairment, but doxycycline does not accumulate in the serum because its gastrointestinal excretion increases.14 During hemodialysis, only about 10% of the drug is removed.15 With hepatic impairment, there is not much pharmacokinetic data. Unlike tetracycline and minocycline, doxycycline does not appear to cause hepatitis.16 Like tetracycline and doxycycline, minocycline is primarily administered orally. It comes in 50-mg, 75-mg, and 100-mg capsules and tablets, as well as a 50 mg/5 mL suspension. There are also 45-mg, 90-mg, and 135-mg extended-release tablets. The usual adult dosage is 200 mg followed by 100 mg every 12 hours. The dosage is the same if minocycline is given intravenously. Each dose should be given over 30 to 60 minutes and must be dissolved in 500 to 1000 mL of glucose or saline.17 Like tetracycline and doxycycline, minocycline is pregnancy category D and should also be avoided in children. If minocycline needs to be given to children, the pediatric dose is 4 mg/kg followed by 2 mg/kg every 12 hours. With impaired renal function, various studies have shown contrasting results. Minocycline does not accumulate in the serum in patients with renal failure, and excretion in such patients is not significantly reduced.18,19 This is because only a small amount of drug is eliminated in the urine. Other reports have found that minocycline can exacerbate preexisting renal insufficiency and possess a prolonged serum half-life, which is directly related to the severity of any renal insufficiency.20,21 With hemodialysis and peritoneal dialysis, minocycline pharmacokinetics are not significantly altered.18,22 In cirrhotic patients, no changes for minocycline are observed.22 The absorption of the tetracyclines occurs primarily in the stomach and proximal small bowel. Tetracycline has a bioavailability of 77% to 88%, and maximum serum concentrations are reached 2 to 4 hours after standard doses with a serum half-life of 7 hours.2,23 Doxycycline is almost completely absorbed in the duodenum and has a prolonged serum half-life of 12 to 16 hours with peak serum levels usually achieved 2 to 3 hours after administration.10 Minocycline is almost completely absorbed in the stomach, duodenum, and jejunum. It peaks in serum after 2 hours and also has a prolonged half-life of 16 hours after the first dose, as well as up to 21 hours after repeated doses.17,20,24 Doxycycline and minocycline absorption are not significantly altered by the administration of food, with levels decreased by less than 20%.25,26 In contrast, tetracycline absorption decreases by approximately 50% when administered with food.26 Multivalent cations (such as aluminum, calcium, iron, and magnesium) chelate with the tetracyclines, resulting in a decrease in drug absorption by 50% to 90%. An interval of 3 hours between the ingestion of tetracyclines and cations prevents this interaction.27 The tetracyclines penetrate well into various body fluids and tissues. Protein binding varies between drugs. Doxycycline is the highest protein-bound drug, estimated between 82% and 93%, followed by minocycline at 70% to 80% and tetracycline at 24% to 65%.3,13,17,28 Tissue and fluid penetration differs on the basis of how lipid soluble each compound is. Doxycycline has been reported to be five times as lipophilic as tetracycline, and minocycline is 10 times more lipophilic than tetracycline.29,30 Tetracycline penetrates readily into pleural, ascitic and synovial fluids and placental-cord serum.2 In maxillary sinus secretions, tetracycline concentrations can nearly equal serum concentrations with repeated dosing.31 However, tetracycline can only be detected in low concentrations in saliva and tears. Sputum levels of tetracycline in the range of 0.4 to 2.6 µg/mL have been detected after oral dosing of 250 mg every 8 hours.32 Cerebrospinal fluid (CSF) levels of tetracycline are about 10% of the simultaneous serum concentration.2 Tetracycline penetrates readily into breast milk but chelates with milk, thus lowering its bioavailability.33 Doxycycline concentrates in the bile with 10 to 25 times greater levels than the serum.34 In thoracic duct lymph and peritoneal fluid, doxycycline concentrations are about 75% of serum levels.35 In the prostate, doxycycline concentrations are up to 60% of serum.36 After an IV dose of 200 mg was given to patients with pleurisy, pleural fluid levels of doxycycline were 25% of serum levels after 2 hours.37 Blister fluid model studies have shown that interstitial fluid concentrations of doxycycline are 54% of serum levels.38 Lower levels of doxycycline have been noted in sputum (8% to 25%), as well as in bone, skin, subcutaneous fat, and tendon tissue.39–41 In the CSF, doxycycline penetration has been studied in both neuroborreliosis and neurosyphilis. In patients with Lyme borreliosis treated with doxycycline 200 mg orally every 12 hours, CSF penetration was noted to be 15% with a concentration of 1.1 µg/mL. At a dose of 100 mg orally every 12 hours, the CSF concentration was only 0.6 µg/mL, suggesting treatment should be with the higher dose.42 In five patients with latent or neurosyphilis receiving 200 mg orally every 12 hours, doxycycline CSF penetration was 26% with a concentration of 1.3 µg/mL, suggesting doxycycline as an alternative to penicillin.43 Like tetracycline, doxycycline penetrates readily into breast milk with levels up to 40% of plasma.33 Because it is less bound to calcium than the other tetracyclines, an infant being breast-fed is at greater risk of side effects. Because minocycline is the most lipophilic of the tetracyclines, it penetrates the most readily into tissues and bacterial cells.44 The highest concentrations are found in the thyroid, lung, gastrointestinal tract, liver, gallbladder, and bile. Other tissues with concentrations higher then serum levels are the prostate, uterus, ovaries, fallopian tubes, breast, skin, tonsils, maxillary sinuses, and eyes.17,22,30,45,46 Minocycline penetrates into CSF better than the other tetracyclines but is only able to achieve low levels.18,22,30 Minocycline can gain higher concentrations in saliva than other tetracyclines, likely explaining why it is effective at eliminating meningococcal carriage.47 Like tetracycline, bioavailability in breast milk is low due to chelation with milk.33 The tetracyclines are each eliminated slightly differently. Tetracycline is eliminated primarily in the kidneys, and about 30% to 60% of an oral dose is excreted in the urine.2,3 The drug accumulates with renal insufficiency, which is why it should be avoided in such patients. About 20% to 60% is eliminated in feces.2 With doxycycline, usually less than 30% of an oral dose is renally excreted.3,48,49 However, unlike tetracycline, fecal excretion is increased in the setting of renal impairment and prevents accumulations of the drug. As much as 90% of doxycycline can be excreted in feces. Minocycline is extensively metabolized in the liver to produce at least six inactive metabolites. Only about 4% to 19% is eliminated by the kidneys, and 20% to 34% is excreted in the feces.19,22,30,50 The tetracyclines as a class have a wide spectrum of antimicrobial activity. Similar susceptibility patterns are displayed among the nonglycylcycline members. They have activity against many aerobic and anaerobic gram-positive and gram-negative bacteria, as well as intracellular organisms and protozoan parasites. Because they are bacteriostatic and many organisms have developed resistance over the years, they have been replaced by other antibiotics as first-line therapy in the majority of cases. When interpreting minimal inhibitory concentration (MIC) results, tetracycline is generally used as the class representative. However, there may be times when specific testing for minocycline or doxycycline is warranted. Most bacterial organisms are considered susceptible to tetracycline when the MIC is 4 µg/mL or less. At 8 µg/mL, tetracycline is considered intermediately active. Resistance is defined as 16 µg/mL or greater. Haemophilus influenzae and Streptococcus pneumoniae have lower MIC breakpoints, with susceptible, intermediate, and resistant set at 2 µg/mL or less, 4 µg/mL, and 8 µg/mL or more, respectively. Even lower is Neisseria gonorrhoeae, with susceptible, intermediate, and resistant set at 0.25 µg/mL, 0.5 to 1 µg/mL, and 2 µg/mL or more, respectively.3 Tetracyclines possess broad activity against gram-positive organisms, including community-acquired Staphylococcus aureus and Streptococcus pneumoniae. Data from the SENTRY Antimicrobial Surveillance Program from 1997-1999 evaluated the susceptibilities of 15,439 patients infected with S. aureus and 6350 infected with coagulase-negative Staphylococcus species (CoNS).51 Samples were taken from various parts of the globe. In the United States, 94.2% of methicillin-susceptible S. aureus (MSSA) strains and 83.7% of methicillin-resistant S. aureus (MRSA) strains were susceptible to tetracycline. Results were similar in isolates from Canada, whereas those obtained from Latin America, Europe, and the Western Pacific had much lower MRSA susceptibilities to tetracycline, 17.8% to 41.4%. In the United States, CoNS was susceptible to tetracycline in 82.9% of the oxacillin-sensitive strains and 79.6% of the oxacillin-resistant strains. These findings were similar to the other regions.51 More recent studies have also shown that the majority (80%) of MRSA isolates were susceptibile to doxycycline and minocycline.52 Reports of S. pneumoniae resistance to doxycycline range geographically from 2% to more than 20%, limiting their use for severe pneumococcal infections.10,53–55 Resistance may be more than 60% in penicillin-resistant strains.55 Among Enterococcus spp., surveillance data from 1997-1999 observed resistance rates approaching 50% in North America, 60% in Europe, and 60% to 70% in Asia-Pacific.56 Actinomyces israelii has shown susceptibility to tetracycline,57 and Listeria monocytogenes58 and Bacillus anthracis59,60 have shown susceptibility to both tetracycline and doxycycline.59,60 As a class, tetracyclines also have a broad range of activity against gram-negative organisms, although they are infrequently used against Enterobacteriaceae. Doxycycline has shown good activity against Yersinia pestis and has more activity than tetracycline.61,62 Among Campylobacter jejuni and Campylobacter coli strains, isolates that have resistance to ciprofloxacin tend to also be resistant to doxycycline.63 Tetracyclines are useful in the treatment of Vibrio spp. in a wide range of clinical conditions from food-borne gastroenteritis to necrotizing skin and soft tissue infections.64–66 In a recent Spanish study with Acinetobacter baumannii, doxycycline had activity against only 30% to 40% of strains tested, while minocycline was active against 70% and tigecycline was active against 76%.67 Doxycycline has excellent activity against Burkholderia pseudomallei with susceptibility rates above 96%, along with low rates or resistance developing during treatment.68 Stenotrophomonas maltophilia is highly susceptible to doxycycline,69 which also exhibits activity against Aeromonas hydrophila.70 Anaerobes in the gastrointestinal tract, including the Bacteroides fragilis group, have shown susceptibility to doxycycline, but with higher MIC breakpoints.71 Brucella spp. and Bartonella spp. are also highly susceptible to doxycycline, with low MIC values.72,73 Among the Mycoplasma spp., tetracyclines are highly active against Mycoplasma pneumoniae. Mycoplasma hominis is usually susceptible, while Mycoplasma genitalium is often doxycycline resistant.74–76 Legionella pneumophila is susceptible to doxycycline in vitro, but this is dependent on the inoculum size because Legionella is intermediately susceptible to doxycycline with larger inocula.77 Chlamydia pneumoniae and Chlamydia psittaci have also been shown to be susceptible to doxycycline.78,79,80 Chlamydia trachomatis is typically susceptible to the tetracyclines, although susceptibility testing is not standardized.79,81 The tetracyclines play a prominent role in the treatment of spirochetes. Doxycycline is the mainstay treatment of Lyme disease caused by Borrelia burgdorferi.82 In a recent study looking at doxycycline, tetracycline, and tigecycline activity against B. burdorferi, tigecycline had the lowest MIC90 (≤0.016 mg/L) versus 0.25 mg/L for both doxycycline and tetracycline.83 For Leptospira spp., doxycycline has been a mainstay of treatment as well.84 Tetracycline has been shown to have activity against Treponema pallidum.85 Among Rickettsiae spp., tetracyclines display activity against the spotted fever group, scrub typhus, and epidemic typhus. Doxycycline has been shown to be the most active antimicrobial agent.86,87 Doxycycline has also shown to be highly active against Anaplasma phagocytophilum, Ehrlichia chaffeensis, and Ehrlichia canis.88 Among mycobacteria, tetracyclines have activity among different species. Against the rapidly growing bacteria, doxycycline has been reported to have activity against 41% to 56% of Mycobacterium fortuitum strains. Less activity is seen against Mycobacterium chelonae (8% to 26%) and Mycobacterium abscessus (4% to 8%).89–91 Similar results are seen with minocycline, although minocycline appears to be more active against M. chelonae and M. fortuitum. Minocycline also appears to have more activity against Mycobacterium marinum than doxycycline and has activity against Mycobacterium kansasii as opposed to doxycycline.91–93 All of the tetracyclines show poor in vitro activity against Mycobacterium avium complex.91 More recently, interest in using doxycycline against Mycobacterium tuberculosis has developed. A Russian study that evaluated second-line agents with activity against multidrug-resistant M. tuberculosis found doxycycline to be active against 92.6% of the 68 isolates tested.94 Doxycycline has also been shown to decrease matrix metalloproteinase activity in a cellular model and suppress mycobacterial growth in vitro and in guinea pigs.95 Among the Nocardia asteroides complex, minocycline appears to have the greatest activity against these and a large number of Nocardia farcinica isolates.10,96 Minocycline has also been shown to be more active than tigecycline with an MIC90 of 2 µg/mL.97
Tetracyclines, Glycylcyclines, and Chloramphenicol
Tetracyclines
Historical Overview and Classification
GENERIC NAME
MAJOR BRAND NAME (COMPANY)
PREPARATION
USUAL ADULT ORAL DOSAGE
NOTES
Short-Acting
Oxytetracycline
Terramycin (Pfizer)
Capsules: 125, 250 mg
500 mg q6h
No longer widely used
Syrup: 125 mg/5 mL
Tetracycline HCl
—
Capsules: 250, 500 mg
500 mg q6h
Syrup: 125 mg/5 mL
Intermediate-Acting
Demeclocycline HCl
Declomycin (Wyeth)
Tablets: 150, 300 mg
150 mg q6h or 300 mg q12h
Used for syndrome of inappropriate antidiuretic hormone secretion
Long-Acting†
Doxycycline
Vibramycin (Pfizer)
Capsules (hyclate): 50, 100 mg
Tablets: 50, 100 mg
200 mg (or 100 mg q12h) for first day, then 100 mg q12h
Syrup (calcium): 50 mg/5 mL
Syrup (monohydrate): 25 mg/5 mL
Minocycline
Minocin (Wyeth)
Capsules and tablets: 50, 100 mg
200 mg, then 100 mg q12h
Suspension: 50 mg/5 mL
Solodyn ER tablets (Medicis)
Extended-release tablets 45, 90, 135 mg
1 mg/kg/day
Long-Acting, Third-Generation
Tigecycline
Tygacil (Wyeth)
Vial: 50 mg lyophilized (reconstituted to 10 mg/mL)
100 mg, then 50 mg q12h
IV
Structure and Mechanism of Action
Pharmacology
Administration and Dosing
Tetracycline
Doxycycline
Minocycline
Absorption and Bioavailability
Drug Distribution
Drug Elimination
Antimicrobial Activity
Gram-Positive Bacteria
Gram-Negative Bacteria
Atypical Bacteria
Spirochetes and Rickettsiae
Mycobacteria and Nocardia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tetracyclines, Glycylcyclines, and Chloramphenicol
26