James H. Diaz Keywords anaplasmosis; argasid ticks; Babesia; babesiosis; Borrelia; borreliosis; ehrlichiosis; Francisella; ixodid ticks; Lyme disease; rickettsialpox; Rocky Mountain spotted fever; tick paralysis; tick-borne coltiviruses; tick-borne encephalitis viruses; tick-borne hemorrhagic fever viruses; tick-borne relapsing fever viruses; tick-borne rickettsioses; ticks; tularemia
Ticks, Including Tick Paralysis
Ticks are the most competent and versatile of all arthropod vectors of zoonotic infectious diseases for several reasons. First, ticks are not afflicted by most of the microorganisms that they may transmit or the paralytic salivary toxins that they may transfer during blood-feeding. Second, and unlike mosquitoes, ticks can transmit the broadest range of infectious microbes among all arthropods, including bacteria, viruses, and parasites. In addition, tick-transmitted coinfections appear to be increasing and complicate differential diagnosis and antimicrobial treatment. Third, ticks can vertically transmit infectious microorganisms congenitally to their offspring of both genders (transovarian transmission) and then disseminate carrier state infections among all generational growth stages (trans-stadial transmission). Tick-borne infectious diseases can also be transmitted to humans by blood transfusions and organ transplants, and babesiosis, a tick-borne infection caused by malaria-related parasites, can be transmitted congenitally. Fourth, ticks have capitalized on many competitive advantages afforded them by evolving changes in climate and human lifestyle, including the following: wider geographic distributions and longer active breeding and blood-feeding seasons as a result of increases in global mean temperatures and humidity; greater abundance of wild animal reservoir hosts no longer effectively controlled, especially deer, rabbits, and rodents; greater residential construction in recently cleared woodlands adjacent to pastures and yards frequented by wildlife, domestic animals, and humans; and more vacation and leisure-time activities enjoyed by humans and their pets during prolonged tick host-questing and blood-feeding seasons from earlier springs through later falls and milder winters.1 In short, ticks of all ages and both genders may remain infectious for generations without having to reacquire infections from host reservoirs and environmental and behavioral changes now place humans and ticks together outdoors for longer periods for tick breeding, blood-feeding, and infectious disease transmission.
Tick Biology, Behavior, and Taxonomy
With the exception of toothed hypostomes for blood-feeding and clawless palps, adult ticks resemble large mites with eight legs and disk-shaped bodies.2 There are four stages in the tick life cycle—egg, six-legged larva, nymph, and adult. Ticks are classified into three families: the Ixodidae, or hard ticks; the Argasidae, or soft ticks; and the Nuttalliellidae, a much lesser known family, with characteristics of both hard and soft ticks.2 Ixodid ticks have a hard dorsal plate or scutum, which is absent in the soft-bodied, argasid ticks. Ixodid ticks also exhibit more sexual dimorphism than argasid ticks, with both genders looking alike. However, all blood-fed ticks, especially females, are capable of enormous expansion and engorged ixodid females are often confused with engorged argasid females. Although ticks from all families may serve as disease vectors, the ixodid or hard ticks are responsible for most tick-borne diseases in the United States.
Ixodid ticks have mouth parts that are attached anteriorly and visible dorsally. They live in open exposed environments, such as woodlands, grasslands, meadows, and scrub brush areas. Argasid ticks are leathery and have subterminally attached mouth parts that are not visible dorsally. Argasid ticks prefer to live in more sheltered environments, including animal nests, caves, crevices, woodpiles, and uninhabited rural cabins. All ticks feed by cutting a small hole in the host’s epidermis with their chelicerae and then inserting their hypostomes into the cut, with blood flow maintained by salivary anticoagulants.2 Ticks are attracted to warm-blooded hosts by vibration and exhaled carbon dioxide. Ixodid ticks actually “quest” for hosts by climbing onto vegetation with their forelegs outstretched; waiting to embrace passing hosts (Fig. 298-1). Ticks spend relatively short periods of their lives mating and blood-feeding on hosts: soft ticks feed rapidly for hours and then drop off, whereas hard ticks blood-feed for days (6 to 12) before dropping off for egg laying.
Epidemiology of Tick-Borne Infectious Diseases
Tick-borne infectious diseases have challenged researchers and physicians since Dr. Howard T. Ricketts identified the wood tick, Dermacentor andersoni, as the vector of Rocky Mountain spotted fever (RMSF) in 1906 and firmly established the insect vector theory of infectious disease transmission.3 The emergence and recognition of Lyme disease in the early 1970s in the United States, whose causative agent, the spirochete Borrelia burgdorferi, was not identified until 1982, sparked renewed interest in tick-borne diseases in the United States and Europe (Fig. 298-2).4
By the early 1990s, Lyme borreliosis had become the most common arthropod-borne infectious disease in the United States and Europe.5 Since the 1970s, every decade now describes emerging or rediscovered tick-borne infectious disease and new vectors for previously described tick-borne diseases, such as RMSF.6 These latest discoveries have been spawned by new immunodiagnostic technologies, especially by nucleic acid identification technologies, particularly the polymerase chain reaction (PCR) assay.
By the 1980s and 1990s, the causative agents of the ehrlichioses were stratified as newly emerging, Rickettsia-like species, and later (2001) were completely reorganized into separate genera, Ehrlichia and Anaplasma.7,8 In 1997, Kirkland and colleagues described a new erythema migrans–like rash illness in North Carolina, a nonendemic region for Lyme disease, transmitted by the lone star tick, Amblyomma americanum (see Fig. 298-1).9 This new borreliosis would soon be named the southern tick-associated rash illness (STARI) or Masters’ disease, but its causative agent, B. lonestari, a new Borrelia species, would not be identified until 2004 (see Fig. 298-2).10,11
By 2004, ticks were recognized as the most common vectors of all arthropod-borne infectious diseases in Europe, five new spotted fever–causing rickettsiae were described, four new subspecies of the Lyme disease–causing B. burgdorferi complex were identified, a new relapsing fever Borrelia species was isolated, and anaplasmosis was exported to Europe from the United States.12 In a seemingly unending era of new discoveries in tick-transmitted diseases, another new and unanticipated vector for RMSF, Rhipicephalus sanguineus, the brown dog tick, was identified in the United States in 2005 (Fig. 298-3).13
In 2011, the first human cases of relapsing fever caused by tick-transmitted Borrelia miyamotoi were reported from Russia, and by 2013, 1% to 3% of surveyed residents of New England states where Lyme disease is endemic were seropositive for prior B. miyamotoi infection.14 In 2009, a new pathogenic Ehrlichia species in addition to endemic Ehrlichia chaffeensis and Ehrlichia ewingii was identified in four febrile patients in Minnesota or Wisconsin and presumed to be related to Ehrlichia muris.15
Because most tick-borne diseases are caused by obligate intracellular organisms, many of which infect erythrocytes, granulocytes, or vascular endothelial lining cells, many tick-borne infections may also be transmitted congenitally (e.g., babesiosis) and by blood product transfusions and organ transplants. Blood product–transmitted infections have now been described for the tick-borne rickettsial diseases (including Q fever), babesiosis, and ehrlichiosis. In 2008, the Centers for Disease Control and Prevention (CDC) reported the first case in which transfusion transmission of Anaplasma phagocytophilum, the tick-borne causative agent of anaplasmosis (formerly, human granulocytic ehrlichiosis [HGE]) was confirmed microscopically and serologically by testing of both the recipient and donor.16
Today, the seroprevalence of tick-borne diseases is increasing significantly among blood and organ donors in the United States, combined tick-transmitted coinfections have been described in regional U.S. populations, and an unexplained increase in the virulence of tick-borne infectious diseases has been described in the United States (RMSF), Europe, and North Africa (Mediterranean spotted fever) and Australia (Queensland tick typhus). Several tick-borne infectious diseases have now been reclassified by the CDC as potential biologic terrorism agents, including the following: Francisella tularensis (tularemia), a category A agent (highly likely microorganism to be weaponized); Coxiella burnetii (Q fever), a category B agent (less likely to be weaponized); and the tick-borne encephalitis and hemorrhagic fever viruses, category C agents (least likely to be weaponized). In the future, the tick-transmitted infectious diseases will increase in prevalence over wider distributions at higher altitudes in a warmer world. Unexpected tick vectors of emerging infections caused by obligate intracellular microorganisms will continue to be discovered as people spend more leisure times outdoors in temperate climates in tick-preferred ecosystems.
Tick-Borne Bacterial Infections
Spirochetal Infections (Borrelioses)
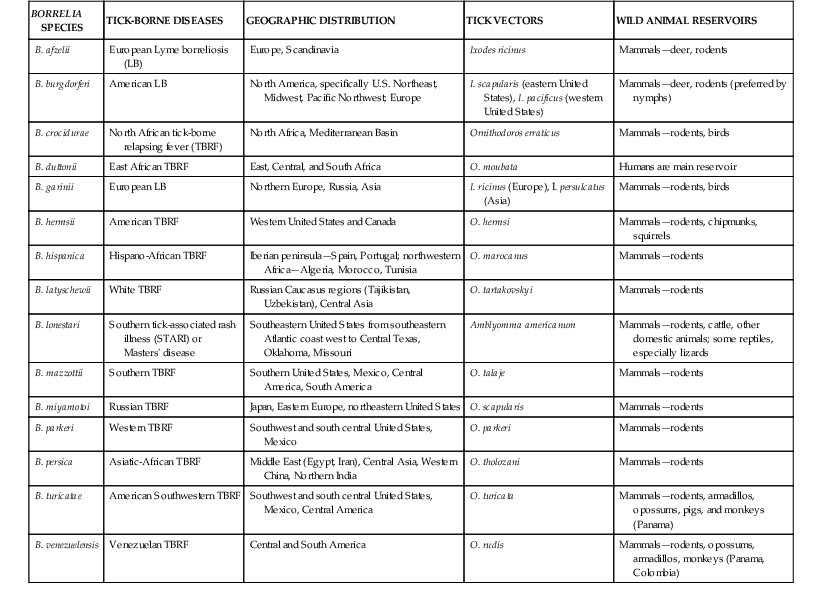
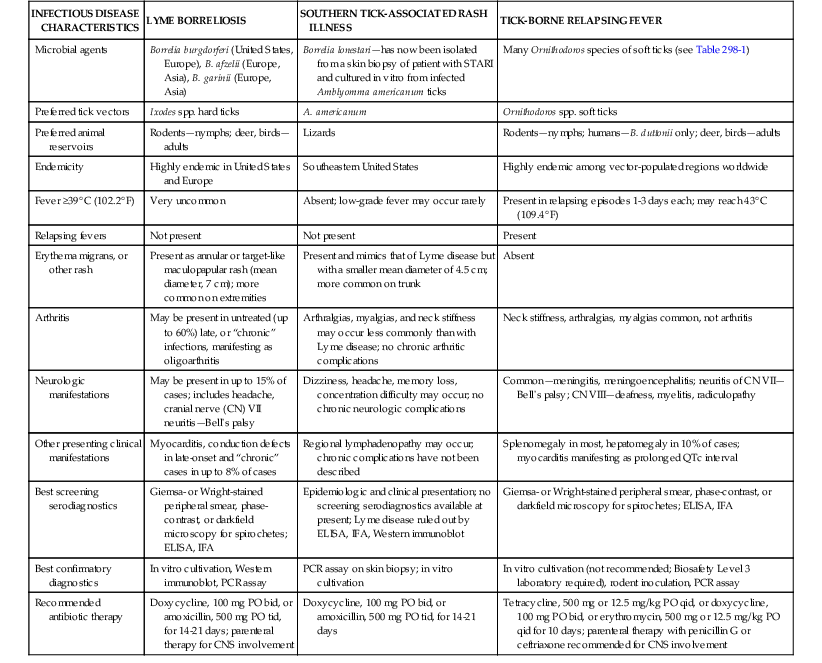
The borrelioses are a large group of tick-borne spirochetal diseases caused by several species of Borrelia, with unique geographic distributions, tick vectors, and host animal reservoirs (Table 298-1). The borrelioses are stratified into three separate epidemiologic and clinical presentations—Lyme borreliosis, STARI, and the tick-borne relapsing fevers (Table 298-2).
TABLE 298-2
Clinicopathophysiologic Comparison of Lyme Borreliosis, Southern Tick-Associated Rash Illness (STARI), and Tick-borne Relapsing Fever
| INFECTIOUS DISEASE CHARACTERISTICS | LYME BORRELIOSIS | SOUTHERN TICK-ASSOCIATED RASH ILLNESS | TICK-BORNE RELAPSING FEVER |
| Microbial agents | Borrelia burgdorferi (United States, Europe), B. afzelii (Europe, Asia), B. garinii (Europe, Asia) | Borrelia lonestari—has now been isolated from a skin biopsy of patient with STARI and cultured in vitro from infected Amblyomma americanum ticks | Many Ornithodoros species of soft ticks (see Table 298-1) |
| Preferred tick vectors | Ixodes spp. hard ticks | A. americanum | Ornithodoros spp. soft ticks |
| Preferred animal reservoirs | Rodents—nymphs; deer, birds—adults | Lizards | Rodents—nymphs; humans—B. duttonii only; deer, birds—adults |
| Endemicity | Highly endemic in United States and Europe | Southeastern United States | Highly endemic among vector-populated regions worldwide |
| Fever ≥39° C (102.2° F) | Very uncommon | Absent; low-grade fever may occur rarely | Present in relapsing episodes 1-3 days each; may reach 43° C (109.4° F) |
| Relapsing fevers | Not present | Not present | Present |
| Erythema migrans, or other rash | Present as annular or target-like maculopapular rash (mean diameter, 7 cm); more common on extremities | Present and mimics that of Lyme disease but with a smaller mean diameter of 4.5 cm; more common on trunk | Absent |
| Arthritis | May be present in untreated (up to 60%) late, or “chronic” infections, manifesting as oligoarthritis | Arthralgias, myalgias, and neck stiffness may occur less commonly than with Lyme disease; no chronic arthritic complications | Neck stiffness, arthralgias, myalgias common, not arthritis |
| Neurologic manifestations | May be present in up to 15% of cases; includes headache, cranial nerve (CN) VII neuritis—Bell’s palsy | Dizziness, headache, memory loss, concentration difficulty may occur; no chronic neurologic complications | Common—meningitis, meningoencephalitis; neuritis of CN VII—Bell’s palsy; CN VIII—deafness, myelitis, radiculopathy |
| Other presenting clinical manifestations | Myocarditis, conduction defects in late-onset and “chronic” cases in up to 8% of cases | Regional lymphadenopathy may occur; chronic complications have not been described | Splenomegaly in most, hepatomegaly in 10% of cases; myocarditis manifesting as prolonged QTc interval |
| Best screening serodiagnostics | Giemsa- or Wright-stained peripheral smear, phase-contrast, or darkfield microscopy for spirochetes; ELISA, IFA | Epidemiologic and clinical presentation; no screening serodiagnostics available at present; Lyme disease ruled out by ELISA, IFA, Western immunoblot | Giemsa- or Wright-stained peripheral smear, phase-contrast, or darkfield microscopy for spirochetes; ELISA, IFA |
| Best confirmatory diagnostics | In vitro cultivation, Western immunoblot, PCR assay | PCR assay on skin biopsy; in vitro cultivation | In vitro cultivation (not recommended; Biosafety Level 3 laboratory required), rodent inoculation, PCR assay |
| Recommended antibiotic therapy | Doxycycline, 100 mg PO bid, or amoxicillin, 500 mg PO tid, for 14-21 days; parenteral therapy for CNS involvement | Doxycycline, 100 mg PO bid, or amoxicillin, 500 mg PO tid, for 14-21 days | Tetracycline, 500 mg or 12.5 mg/kg PO qid, or doxycycline, 100 mg PO bid, or erythromycin, 500 mg or 12.5 mg/kg PO qid for 10 days; parenteral therapy with penicillin G or ceftriaxone recommended for CNS involvement |
CNS, central nervous system; ELISA, enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; PCR, polymerase chain reaction.
Lyme borreliosis (LB) or Lyme disease is now the most common tick-borne infectious disease in the Northern Hemisphere and the most common arthropod-borne infectious disease in the United States.5,17 In the United States, LB is caused by Borrelia burgdorferi (sensu stricto), first identified as a novel bacterial spirochete in 1982, and transmitted to humans by Ixodes spp. hard ticks in U.S. regional pockets, specifically the Northeast (I. scapularis), upper Midwest (I. scapularis), and Pacific Coast (I. pacificus; Fig. 298-4; see also Fig. 298-2). Although B. burgdorferi is the sole agent of LB in the United States and has been exported to Europe, most cases of LB in Europe and northern Asia are caused by B. afzelii and B. garinii (see Table 298-1). Collectively, the three Borrelia species are often referred to as B. burgdorferi (sensu lato). Ticks usually acquire Borrelia infections as larvae or nymphs by blood-feeding on small reservoir hosts, most commonly birds and rodents, and may transmit LB to humans during blood-feeding, which may go unnoticed (see Fig. 298-4). Borrelia organisms are further maintained in nature as infected adult Ixodes ticks blood-feed on larger mammals, especially deer.
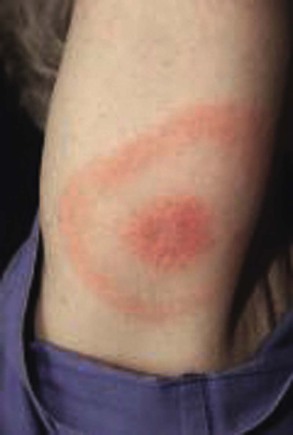
Unlike argasid or soft ticks, Ixodes ticks prefer temperate ecotonal zones of canopied forests abutting cleared scrub or grasslands and transmit B. burgdorferi to humans during outdoor exposures in such habitats. Because Borrelia spirochetes must migrate from the tick’s midgut to the salivary gland during blood-feeding, tick attachments for less than 24 hours rarely result in LB in humans.18 After an incubation period of 1 to 2 weeks, the hallmark of spirochete transmission manifests as solitary erythema migrans, a maculopapular erythematous rash with a bull’s eye pattern, at the site of tick attachment (Fig. 298-5). Erythema migrans also occurs in STARI at the site of Amblyomma americanum or lone star tick attachment and results from the subcutaneous centrifugal movement of the spirochetes from the bite sites to the central circulation and target organs (see Fig. 298-5).
In a meta-analysis of 53 longitudinal studies of LB in the United States and Europe, Tibbles and Edlow have reported that many patients do not recall a tick bite (74% in the United States, 36% in Europe), constitutional symptoms of low-grade fever (<39° C [102.2° F]) and headache are common but nausea and vomiting are rare, and a solitary erythema migrans lesion is the most common initial presentation of LB (81% in the United States, 88% in Europe).19 Although deaths from LB are rare, the greatest morbidity from target organ damage in LB occurs in patients with prolonged or untreated infections, with 5% to 8% developing cardiac manifestations, 15% to 20% developing neurologic manifestations, and 40% to 60% developing chronic arthritis.18–20 However, if LB is recognized and treated early in the erythema migrans stage, cure rates will exceed 90%, late manifestations of chronic arthritis will be avoided, and outcomes will be excellent (see Table 298-2).
In LB-untreated patients, recurrent attacks of chronic arthritis were formerly referred to as chronic LB.20,21 Later, as all patients in whom LB was diagnosed were treated with antibiotics, synovitis persisting for months to years after initial treatment was renamed “antibiotic-refractory arthritis.”21 Antibiotic-refractory arthritis was attributed to a combination of retained spirochetal antigens and postinfectious autoimmune reactions.21 However, recent investigations by Nadelman and co-workers have now dispelled the former concept of chronic persisting B. burgdorferi infections and have demonstrated that repeat episodes of pathognomonic erythema migrans in appropriately treated LB patients were due to reinfections and not to recurrences.22,23
The Jarisch-Herxheimer reaction (JHR), an inflammatory cytokine-mediated reaction to dying spirochetes with a worsening of presenting symptoms, vasodilatation, and myocardial dysfunction, may occur during antibiotic treatment for LB but is more common after antibiotic therapy for tick-borne relapsing fevers.18,24 There have been no reported deaths from JHR during antibiotic therapy for LB, and the very rare case fatalities from LB have been attributed to cardiac conduction abnormalities from myocarditis in untreated cases.18
First recognized in 1998, STARI manifests initially as erythema migrans, as in LB, but occurs in regions in which B. burgdorferi is not endemic and follows the prolonged attachment of blood-feeding lone star ticks, Amblyomma americanum, more abundant in the southeastern and south central United States (see Figs. 298-1 and 298-5).9,18 Patients who are bitten by lone star ticks may develop LB-like erythema migrans rashes and occasionally develop milder constitutional symptoms than in LB, including fever, headache, fatigue, and generalized myalgias. However, unlike LB, STARI is not a reportable infectious disease and has no diagnostic serologic tests, such as enzyme-linked immunoassays (ELISAs), immunofluorescent assays (IFAs), and Western immunoblot assays. In addition, a microbiologic analysis of skin biopsy specimens obtained from the rashes of 30 patients in Missouri with clinical diagnoses of STARI failed to detect B. lonestari, suggesting that STARI could be caused by other pathogens.25 Because some patients have recovered from STARI without antibiotic treatment and there have been no long-term sequelae reported in STARI cases, some have questioned whether antibiotic therapy is indicated in STARI. Because distinguishing STARI from LB may be difficult, Wormser and co-workers have recommended that the differential diagnosis rely on a combination of regional exposures, clinical presentations, serologic results, and potential for long-term sequelae based on their comparison of LB cases from New York and STARI cases from Missouri.25 The investigators noted that the timing of rash onset was shorter (6 days) in STARI compared with LB (10 days) and that STARI patients were less likely to be symptomatic than LB patients.25 In addition, the STARI rash was more often circular with central clearing than the LB rash.25 Most authorities recommend antibiotic therapy for STARI with oral doxycycline or amoxicillin following the same regimen as for LB to cover any missed diagnoses of LB with potential for chronic arthritic and cardiac sequelae (see Table 298-2).25
The tick-borne relapsing fevers (TBRFs) comprise a worldwide group of serious bacterial infections by Borrelia spirochetes after brief, painless, and usually unnoticed bites by Ornithodoros spp. argasid or soft ticks. These ticks prefer indoor living—in cabins, caves, and crevices—and quickly abandon warm-blooded rodent hosts for egg laying (see Table 298-1).24,25 Unlike the ixodid ticks, Ornithodoros ticks feed very briefly, usually for less than 30 minutes, and at night.24,26 Adults can live for as long as 15 to 20 years and survive without blood meals for several years. Transovarian transmission of the TBRF spirochetes occurs commonly among all species and, unlike LB-causing Borrelia species, TBRF spirochetes are already present in the salivary glands at the onset of blood-feeding and do not need time to migrate from the gut to the mouth parts. The wild animal host reservoirs of TBRF are maintained in birds and several mammals, most commonly rodents. The bite of a TBRF-infected tick is painless, and the bite site is marked after a few days by a small red to violaceous papule with a central eschar.18,26 One spirochete is sufficient to initiate TBRF, and the infection rate after a single bite by an infected tick is more than 50%. The incubation period to onset of the first febrile episode is 3 to 12 days.
TBRF is defined clinically by the sudden onset of two or more episodes of high fever (>39° C [102.2° F]) spaced by afebrile periods of 4 to 14 days, with the first febrile episode lasting 3 to 6 days and the relapsing episodes lasting 1 to 3 days each.18,24,26 The first episode ends with a 15- to 30-minute “crisis” with tachycardia, hypertension, hyperpyrexia (as high as 43° C [109.4° F]), and rigors, followed by diaphoresis and defervescence.18,26,27 All febrile episodes are accompanied by nausea, headache, neck stiffness, myalgia, and arthralgia. The relapsing febrile episodes result from the growth of new spirochete populations in the blood to replace those killed by macrophages and cytokines. Most patients have splenomegaly, 10% will have hepatomegaly, and most will have elevated aminotransferase levels, unconjugated bilirubin, and prolonged prothrombin and partial thromboplastin times.24,27 Direct neurologic involvement is more common than in LB and may include cranial nerve neuritis (especially cranial nerves VII and VIII), radiculopathy, and myelopathy. Myocarditis is also more common than in LB; may be complicated by adult respiratory distress syndrome (ARDS), pulmonary edema, and cardiomegaly; and is often fatal. Diagnostic and treatment strategies for TBRF are outlined in Table 298-2.
The JHR is much more common, although rarely fatal, during treatment of TBRF than during treatment of LB and occurs in 30% to 40% of patients with TBRF.18 At present, no prophylactic strategies to reduce the severity of the JHR have proved beneficial or have been adequately tested in multiple clinical trials, including therapy with antipyretics, corticosteroids, or naloxone. Treatment with penicillin instead of tetracycline has a slightly lower risk for causing JHR during antibiotic therapy for TBRF.
Spotted Fever Group Rickettsial Infections
The family Rickettsiaceae contains two genera, the spotted fever–causing genus Rickettsia and the typhus-causing genus Orientia (see Chapters 188 and 193). The rickettsiae may be further stratified clinically into the tick-borne spotted fever group and mouse mite–transmitted rickettsialpox caused by Rickettsia akari (see Chapter 189). The rickettsiae are obligate intracellular, gram-negative bacteria that thrive in ixodid tick salivary glands and are transmitted during blood-feeding. Once injected into the host, rickettsiae are initially distributed regionally via lymphatics, with some species causing marked regional lymphadenopathy (e.g., Rickettsia slovaca). Within 2 to 14 days (mean, 7 days), rickettsiae are disseminated hematogenously to vascular endothelial lining cells of target organs, including the central nervous system (CNS), lungs, and myocardium. Rickettsiae gain entry into host endothelial cells in a Trojan horse–like manner by using their outer membrane proteins (OmpA and OmpB) to stimulate endocytosis. Once within phagosomes, rickettsiae escape to enter the cytosol or nucleus for rapid replication by binary fission, safe from host immune attack. The tick-borne rickettsial diseases that cause spotted fevers (SFs) are compared in a descending order of clinical severity of infection by preferred tick vectors and wild animal reservoirs in Table 298-3.
TABLE 298-3
Spotted Fever Group of Tick-borne Rickettsioses
| RICKETTSIA SPECIES | TICK-BORNE DISEASES | GEOGRAPHIC DISTRIBUTION | TICK VECTORS | WILD ANIMAL RESERVOIRS (MAMMALS) |
| R. rickettsii | Rocky Mountain spotted fever (SF), Brazilian SF | Continental United States, Central America (Costa Rica, Mexico, Panama), South America (Argentina, Brazil) | Amblyomma, Dermacentor, Rhipicephalus spp. | Ungulates, rodents |
| R. conorii | Boutonneuse fever, Mediterranean SF, Israeli SF, Astrakhan SF, Indian tick typhus, Kenyan tick typhus | Mediterranean Basin, Africa, Middle East, Asia | Rhipicephalus spp. | Ungulates, rodents |
| R. sibirica | North Asian tick typhus (Siberian tick typhus) | Africa (Niger, Mali, South Africa), Asia (Russia, China, Mongolia, Pakistan, Kazakhstan, Kirgizia, Tajikistan), Europe (France) | Dermacentor, Haemaphysalis, Hyalomma spp. | Ungulates, rodents |
| R. japonica | Japanese SF | Japan and China | Haemaphysalis spp., Ixodes ovatus | Ungulates, rodents |
| R. australis | Queensland tick typhus | Eastern Australian seaboard from Cairns, Queensland, to Gippsland, Victoria | Ixodes spp., especially I. holocyclus | Rodents |
| R. honei | Flinders Island SF | Southern Australia, Thailand | Aponomma spp. | Rodents |
| R. africae and R. parkeri | African tick bite fever | Sub-Saharan Africa, North America, South America, Caribbean | Amblyomma spp. | Rodents |
| R. slovaca | Tick-borne lymphadenopathy; Dermacentor-borne eschar, lymphadenopathy, or necrosis | Europe | Dermacentor spp. | Ungulates, rodents |
| R. aeschlimannii | Not named at present | Southern Europe, Africa | Hyalomma spp. | Ungulates, rodents |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree