Andrew B. Onderdonk, Wendy S. Garrett
Gas Gangrene and Other Clostridium-Associated Diseases
The genus Clostridium includes more than 200 described species. Members of this genus participate in a variety of invasive and toxigenic processes and can cause disease that is strictly toxin mediated, such as antibiotic-associated colitis (AAC) and foodborne botulism, or they can contribute to invasive infections, including bacteremia, clostridial myonecrosis (gas gangrene), and other suppurative infections resulting from the production of histotoxins and enzymes that destroy soft tissue. Historically, clostridial infections were recognized as discrete clinical syndromes well before the germ theory of disease was proposed. The clinical features of tetanus were well described by some of the earliest medical writers, such as Hippocrates, and the toxic nature of this species was noted as early as the 1870s.1 Clostridia are often isolated as part of a mixed microflora during suppurative infections that occur as a result of fecal or soil contamination of otherwise sterile tissue. Before 1977, the most commonly reported clostridial infections and intoxications were those caused by C. perfringens. Other species, most notably C. tetani in nonimmunized individuals (see Chapter 244) and C. botulinum (see Chapter 246), also generated considerable interest due to the severity and often fatal nature of the intoxications they caused. With the discovery of the etiology of AAC first in an animal model2 and subsequently in humans,3 it soon became clear that in the antibiotic era, C. difficile was the most common clostridial species associated with the human disease formerly called C. difficile–associated diarrhea (CDAD) and currently called C. difficile infection (CDI). Within the hospital setting, CDI has become a significant worldwide nosocomial infection problem,4 resulting in both toxin-mediated diarrheal disease and more fulminant presentations such as pseudomembranous enterocolitis and toxic megacolon. CDI is discussed in detail in Chapter 245. Well-recognized pathogenic members of the genus Clostridium, as well as previously obscure species, continue to participate in a broad array of infectious processes.
Characteristics of Clostridium Spp.
Members of this genus are phenotypically characterized as anaerobic, gram-positive rods that are capable of forming endospores. Clostridium spp. are ubiquitous in nature, found in soils and sediments throughout the world and as members of the intestinal microflora of humans and most other animals. It has been reported that more than 70% of humans are colonized with clostridia at concentrations of 108 to 109 organisms per gram of feces.5 Clostridia can also be isolated as part of vaginal microflora of healthy women,6 although they tend to be transient members of the vaginal microflora, occurring in low numbers as a result of contamination by intestinal microflora rather than as part of the autochthonous community. Most members of this genus are obligate anaerobes, whereas strains of a few species such as C. tertium, C. histolyticum, C. innocuum, and C. perfringens are aerotolerant and can be confused with members of the genus Bacillus during laboratory diagnosis. On the basis of 16S rDNA sequence data, members of the genus Clostridium are part of the phylum Firmicutes, a diverse group of gram-positive organisms including both spore-forming and non-spore-forming genera. On the basis of 16S rDNA sequence analysis, the clostridia can be divided into 11 homology groups, with most of the clinically significant species belonging to homology group 1.7 Traditional classification methods for the clostridia rely on carbohydrate fermentation profiles, detection of short-chain fatty acid end products of fermentation, Gram-stain morphology, colony morphology on agar media, and detection of specific toxins. Although many different species have been isolated from human clinical material, only a small number of species are regularly associated with human disease (Table 248-1).
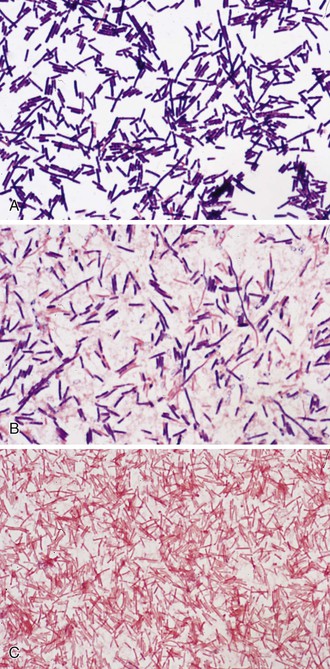
Microscopically, the vegetative cells of Clostridium spp. are rod shaped, often pleomorphic, and found as short chains, clusters, or pairs. The cells of most species have rounded ends. This may vary with some species, which show more pointed ends (C. ramosum). Some species form long chains (C. spiroforme), which may be tightly packed to form coils. Clostridia usually stain gram positively in young cultures (C. perfringens; Fig. 248-1A), with some species losing this staining characteristic in older cultures (C. novyi; see Fig. 248-1B). Species such as C. clostridioforme and C. ramosum rarely show the typical gram-positive appearance and present as gram-variable or gram-negative rods (see Fig. 248-1C). When spores are present, they tend to be ovoid or spherical, with the spore often distending the vegetative cell to produce a club-shaped appearance. Spores may be located centrally, subterminally, or as terminal structures, depending on the species. Spore location is used as part of the identification process. Most clostridia are motile by virtue of peritrichous flagella, with the notable exception of the common clinical isolates, C. perfringens and C. ramosum.7
Clostridium spp. have diverse metabolic pathways and can be saccharolytic, proteolytic, both, or neither. Clostridium spp. are not known to reduce sulfate. The end products of fermentative metabolism are mixtures of short-chain fatty acids and alcohols, a characteristic that can be used for identification purposes in the clinical laboratory. Aerotolerant strains of clostridia do not form spores in the presence of oxygen, they are catalase negative, and they grow more abundantly under anaerobic conditions. Clostridia do not have a complete cytochrome system and are therefore oxidase negative.7 Most strains are catalase and superoxide dismutase negative, although trace amounts of activity have been reported for some species. Clostridia produce a variety of biologically active proteins, including hemolysins, proteolytic enzymes, and other toxins. It is the protein toxins produced by clostridia that account for their importance in human disease. Clostridia produce a greater diversity of toxins than any other genera of bacteria.7 These include neurotoxins, enterotoxins, collagenases, proteases, and other necrotoxins, lecithinases, lipases, DNAses, and neuraminidases. The potency of some of these toxins, such as botulinum neurotoxin (BoNT) and tetanus neurotoxin (TeNT), render them among the most lethal substances yet described; less than 0.2 ng of purified toxin is fatal in mice (see Chapters 244 and 246).
Invasive infections caused by clostridia are invariably due to organisms that are either part of the normal intestinal and vaginal microflora or acquired by a traumatic injury that breaches the skin, which then becomes contaminated with soil, unsanitary water, or fecal material. Intoxications can occur either in response to endogenous toxin production, such as that associated with CDI, or by ingestion of preformed toxins contaminating food, as is the case for noninfant botulism. With the exception of environmental spread of C. difficile within a susceptible population, such as hospitalized patients on broad-spectrum antibiotic therapy and residents in nursing homes, clostridia rarely cause infection through person-to-person contact.
The spores of clostridia account for their persistence in hostile environments and also their exogenous acquisition by humans. In addition to their long-term survival in soil or food, clostridial spores may spread via aerosol transmission as part of naturally occurring dust clouds. C. difficile is of particular concern because this species may be part of the intestinal microflora of an individual or acquired through contact with individuals or contaminated surfaces and equipment within the hospital environment harboring spores. The vegetative cells of clostridia are generally susceptible to routinely used disinfectants, but spores can survive hostile environments, including heat, desiccation, and exposure to many commonly used disinfectants.8–10 This allows pathogenic clostridia to persist in an environment, even following routine disinfection procedures. Methods for eliminating clostridial spores from some environments, such as C. difficile in the hospital setting, include finding methods to promote germination of the spores so that the vegetative cells can be destroyed.8,11
Clostridium spp., particularly members of clusters IV and XIVa derived from the gut microbiota, have recently been shown to have beneficial effects on the immune system. Specifically, consortia of Clostridium spp. promoted accumulation of colonic regulatory T cell development in mice.12 Inoculation of these Clostridium spp. into mice increased their resistance to chemically induced colitis and blunted their allergic responses. This recent study casts this genus in a new light, suggesting its members are not only fearsome pathogens but also may hold therapeutic potential for autoimmune and allergic conditions.
Clostridium perfringens and Clostridial Myonecrosis (Gas Gangrene)
Clostridial myonecrosis, or gas gangrene, is most often caused by a traumatic injury that becomes contaminated with clostridial spores from species that cause myonecrosis, most commonly those of C. perfringens. Throughout history, cases of gas gangrene have increased during times of human conflict due to the numbers of traumatic injuries provoked during violent conflict. Although other clostridial species can and do cause gas gangrene, the prevalence of C. perfringens in such infections marks it as one of the major clostridial pathogens. C. perfringens can be isolated from soil and sediment samples from any geographic region. It is also one of the most common clostridial species isolated from the intestinal tract of humans and other animals. The ability of C. perfringens to cause myonecrosis is due to the production of a variety of protein toxins. Because strains of C. perfringens vary in their ability to produce some of the major toxins, this attribute differentiates this species into strain types (Table 248-2). Epidemiologically, C. perfringens type A is most common in human stool,13 with other types more commonly associated with food poisoning and other enterotoxigenic disease (see following). Gas gangrene, although most often associated with C. perfringens, can be caused by other species, including C. septicum, C. sordellii, C. novyi, C. bifermentans, and C. histolyticum. The common denominator for all of these species is the production of exotoxins, most often lecithinase, that devitalize tissue and promote invasive disease and myonecrosis.
TABLE 248-2
Toxins Produced by Clostridium perfringens
| TOXIN | STRAIN TYPES | BIOLOGIC ACTIVITY |
| α | All strains | Lecithinase |
| β | B and C | Necrotoxin, necrosis of the bowel |
| ε | B and D | Lethal, hemorrhagic |
| ι | E | Adenosine diphosphate ribosylating; lethal |
| cpe enterotoxin | A, C, and D | Cytopathic |
| Neuraminidase | All strains | Hydrolyzes N-acetylneuraminic acid |
| δ | B and C | Hemolysins |
| κ | All strains | Collagenase |
| λ | B, D, and E | Protease |
| μ | All strains | Hyaluronidase |
| ν | All strains | DNAase |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree