Vascular Disease of the Lower Extremities in Diabetes Mellitus: Etiology and Management
Cameron M. Akbari
Frank W. LoGerfo
Problems of the diabetic foot are the most common causes of hospitalization in patients with diabetes, with an annual health care cost of more than $1 billion (1). The prospect of possible amputation terrifies patients with diabetes and justifiably so: diabetes mellitus is a contributing factor in half of all lower extremity amputations in the United States, and the relative risk for leg amputation is 40 times greater among persons with diabetes than among those without diabetes (1,2). Moreover, up to 50% of diabetic amputees will undergo a second leg amputation within 5 years of the initial amputation. Foot ulceration will affect 15% of all individuals with diabetes during their lifetime, with an annual incidence of 3% in patients with diabetes, and is clearly a significant risk factor in the pathway to limb loss (3).
THE DIABETIC FOOT: FUNDAMENTAL CONSIDERATIONS
The principal pathogenic mechanisms in diabetic foot disease are ischemia, neuropathy, and infection; acting together they contribute to the sequence of tissue necrosis, ulceration, and gangrene. Fundamentally, limb salvage strategies are focused on a clear understanding of these abnormalities in patients with diabetes, which subsequently allows for improved prevention and treatment in this population.
Although discussed in greater detail elsewhere in this book, a brief discussion of neuropathy is warranted here, since it afflicts as many as 50% to 60% of all patients (4,5) and is present in more than 80% of diabetic patients with foot lesions (6). Broadly classified as focal and diffuse neuropathies, the latter are more common and include the autonomic and chronic sensorimotor polyneuropathies, which both contribute to foot ulceration.
Sensorimotor neuropathy initially involves the distal lower extremities, progresses centrally, and is typically symmetric. Sensory nerve-fiber involvement leads to loss of the protective sensation of pain, whereas motor-fiber destruction results in small-muscle atrophy in the foot. Consequently, the metatarsals are flexed with metatarsal head prominence and “clawing” of the toes. This causes abnormal pressure points to develop on the bony prominences without sensation, with subsequent erosion and ulceration. Meanwhile, autonomic neuropathy in the foot causes loss of sympathetic tone, which results in increased arteriovenous shunting and inefficient nutrient flow. Autonomic denervation of oil and sweat glands leads to cracking of dry skin, which further predisposes the diabetic foot to skin breakdown and ulceration (7).
The spectrum of infection in diabetic foot disease ranges from superficial ulceration to extensive gangrene with fulminant sepsis. The majority of infections are polymicrobic, with the most common pathogens being staphylococci and streptococci. The more complicated wounds, such as deep ulcers or those with associated abscesses, may harbor anaerobes and gram-negative bacilli. Potential sources of diabetic foot infection include a simple puncture wound or ulcer, the nail plate, and the interdigital web space. Untreated cellulitis can lead to the spread of bacteria along tendon sheaths and to deep-space plantar infection, with eventual destruction of the interosseous
fascia and spread to the foot dorsum. With worsening infection, edema is also commonly seen, which leads to elevated compartmental pressures, with resultant capillary thrombosis and further impairment of nutrient blood flow.
fascia and spread to the foot dorsum. With worsening infection, edema is also commonly seen, which leads to elevated compartmental pressures, with resultant capillary thrombosis and further impairment of nutrient blood flow.
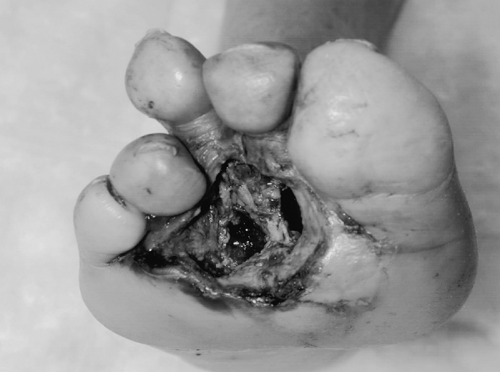
Evaluation for ischemia may begin once the infection has begun to resolve and signs of systemic toxicity have disappeared. The decision to perform arteriography and vascular reconstruction should be made during this waiting period, which is usually no longer than 5 days. Further delays may lead to loss of opportunity to salvage the foot (8). A proper evaluation for underlying vascular disease is essential for limb salvage in patients with diabetic foot ulceration, even when neuropathy and infection are present. All limb salvage efforts will fail unless ischemia has been recognized and corrected (Fig. 67.1). Most important, consideration should be given to the unique pathophysiology of diabetes and vascular disease and the need to tailor treatment decisions in diabetic patients with lower extremity vascular disease.
PATHOPHYSIOLOGY OF VASCULAR DISEASE IN DIABETES MELLITUS
Microvascular Abnormalities
Many of the complications of diabetes may best be characterized as alterations in vascular structure and function, with subsequent end-organ damage and death (9). Specifically, two types of vascular disease are observed in patients with diabetes, a nonocclusive microcirculatory impairment involving the capillaries and arterioles of the kidneys, retina, and peripheral nerves and a macroangiopathy characterized by atherosclerotic lesions of the coronary and peripheral arterial circulation (10,11,12,13). The former is relatively unique to diabetes, whereas the latter lesions are morphologically and functionally similar in nondiabetic and diabetic patients.
One of the greatest impediments to understanding and treating vascular disease in patients with diabetes is the misconception that they have an untreatable occlusive lesion in the microcirculation, which has fostered the belief that arterial reconstruction is futile. This idea originated from an uncontrolled histologic study demonstrating the presence of periodic acid-Schiff-positive material occluding the arterioles in amputated limb specimens from diabetic patients (14). However, subsequent prospective anatomic staining and arterial casting studies (15) have demonstrated the absence of an arteriolar occlusive lesion (16). Further evidence comes from physiologic studies of femoro-popliteal bypass grafts in diabetic and nondiabetic patients in which direct vasodilator administration into these grafts demonstrates a comparable fall in peripheral resistance between the two groups (17), again supporting the absence of a distal occlusive arteriolar lesion. Dispelling the notion of “small-vessel disease” is fundamental to the principles of limb salvage in patients with diabetes, since arterial reconstruction is almost always possible and successful in these patients.
While there is no occlusive lesion in the diabetic microcirculation, other structural changes do exist, most notably thickening of the capillary basement membrane. However, these changes do not lead to narrowing of the capillary lumen, and arteriolar blood flow may be normal or even increased despite these changes (18). Thickening of the capillary basement membrane is a structural change observed in both diabetic retinopathy and nephropathy.
In the diabetic foot, thickening of the basement membrane theoretically may impair the migration of leukocytes and the hyperemic response following injury and thus may increase the susceptibility of the diabetic foot to infection (19,19). Although resting total skin microcirculatory flow is similar in diabetic and nondiabetic patients, the capillary blood flow is reduced in patients with diabetes, indicating a maldistribution and functional ischemia of the skin (21). Moreover, studies of skin microvascular flow have demonstrated a reduced maximal hyperemic response in patients with diabetes, suggesting that a functional microvascular impairment is a major contributing factor to diabetic foot problems (22). All of these changes result in an inability to vasodilate and achieve maximal blood flow following injury.
Diabetes also affects the axon reflex (23). Normally, injury directly stimulates nociceptive C fibers, which results in both orthodromic conduction to the spinal cord and antidromic conduction to adjacent C fibers and other axon branches. One function of this axon reflex is neuropeptide secretion, such as substance P and calcitonin gene-related peptide, which directly and indirectly (through release of histamine by mast cells) causes vasodilation and increased permeability. This neurogenic vasodilatory response is impaired in diabetes, further reducing the hyperemic response when it is most needed—under conditions of injury and inflammation (24)
Endothelial dysfunction is seen in the earliest stages of atherogenesis and is characterized in vivo by an impaired endothelium-dependent vasodilatory response. Normally, endothelium-derived nitric oxide allows for arterial vasodilation in response to either a pharmacologic (e.g., acetylcholine) or a physiologic (e.g., increased shear stress) stimulus. Both type 1 and type 2 diabetes are characterized by an abnormal endothelium-dependent vasodilation (25,26), possibly due to the effects of prolonged hyperglycemia or insulin resistance on both the micro- and macrocirculation (27).
Macrovascular Abnormalities
Lower extremity arterial disease is more common among patients with diabetes than among those without diabetes. The presence of diabetes is associated with a two- to threefold excess risk of intermittent claudication compared with its absence (28). Despite significant advances in the prevention and treatment of peripheral vascular disease, diabetes continues to be the single strongest cardiovascular risk factor for the development of critical leg ischemia and limb loss (29).
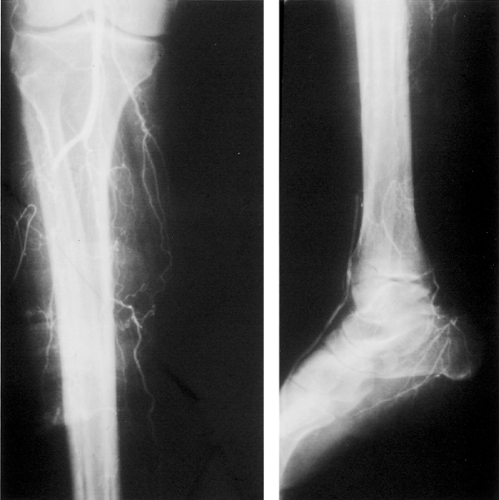
Unlike microvascular disease, which is unique to diabetes and its metabolic alterations, the cause of lower extremity ischemia is similar in diabetic and nondiabetic patients and is due to accelerated atherosclerosis. One notable difference between these populations is the pattern and location of the occlusive atherosclerotic lesion. As noted earlier, there is no evidence for an occlusive lesion at the arteriolar level (“small-vessel disease”) in patients with diabetes. However, patients with diabetes are more likely to have atherosclerotic disease affecting the infrapopliteal (tibial) arteries, with sparing of the foot arteries (30), which allows for successful arterial reconstruction to these distal vessels. Conversely, the superficial femoral or popliteal artery is less likely to be affected by the occlusive process, allowing these vessels to serve as a possible inflow source for bypass grafting (Fig. 67.2).
Because the foot vessels are often patent in the patient with diabetes and because of the success of bypass grafting to these vessels, an appropriate evaluation for ischemia is essential in patients with diabetes. The complex milieu of motor and sensory neuropathy, thickening of the capillary basement membrane, loss of the neurogenic inflammatory response, and the wide spectrum of microcirculatory and endothelial abnormalities all result in a biologically compromised foot. Unless ischemia is recognized and corrected, limb salvage efforts with the diabetic foot will fail, even if infection and neuropathy have been appropriately treated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree