Effects of Diabetes Mellitus on the Digestive System
Hiroshi Mashimo
Roger J. May†
Raj K. Goyal
†Deceased
In 1936, Bargen (1) first described diarrhea and steatorrhea as complications of diabetes. It is now clear that all parts of the digestive system are affected by diabetes, and digestive system dysfunction is an important contributor to the morbidity of this disease (2,3,4). Digestive symptoms related to diabetes are reported to be common. Feldman and Schiller (3) questioned 136 unselected patients at a hospital diabetes clinic about the presence of gastrointestinal (GI) symptoms. Three fourths of all
patients had digestive symptoms. Frequent GI symptoms were constipation (60%), abdominal pain (34%), nausea and vomiting (29%), diarrhea (22%), and fecal incontinence (20%). Moreover, digestive symptoms in persons with diabetes may lead to clinically significant decreases in quality-of-life scores (5). A more recent U.S. national survey of upper GI symptoms, including heartburn, also showed significantly more upper GI symptoms in persons with diabetes (50% vs. 38% in controls) (6). However, it is unclear whether such GI symptoms are significantly different in the diabetic population than in the nondiabetic population except for decreased heartburn and increased use of laxatives (7). Regardless, asymptomatic abnormalities of gut function frequently occur in these patients.
patients had digestive symptoms. Frequent GI symptoms were constipation (60%), abdominal pain (34%), nausea and vomiting (29%), diarrhea (22%), and fecal incontinence (20%). Moreover, digestive symptoms in persons with diabetes may lead to clinically significant decreases in quality-of-life scores (5). A more recent U.S. national survey of upper GI symptoms, including heartburn, also showed significantly more upper GI symptoms in persons with diabetes (50% vs. 38% in controls) (6). However, it is unclear whether such GI symptoms are significantly different in the diabetic population than in the nondiabetic population except for decreased heartburn and increased use of laxatives (7). Regardless, asymptomatic abnormalities of gut function frequently occur in these patients.
PATHOGENESIS OF DIGESTIVE SYSTEM DYSFUNCTION
The digestive system dysfunction in diabetes may result from diabetes itself or, more often, from diabetes-associated complications. Diabetic neuropathy plays an important role in motor and secretory abnormalities in the GI tract, in nausea, vomiting, and the syndrome of abdominal pain. Diabetic angiopathy and vascular complications play a role in the pathogenesis of intestinal ischemia, in the severity and outcome of cholecystitis and biliary tract surgery, and in the nerve and muscle dysfunction of diabetic gastroenteropathy. Defects in immune mechanisms in diabetes are related to an increased incidence of esophageal candidiasis, and decreased resistance to infection accounts for the many pyogenic complications in the digestive tracts of these patients.
Some of the digestive system abnormalities in diabetes may not be causally related to diabetes but may reflect a common association of diabetes with these abnormalities. For example, the increased incidence of gallstones and fatty liver is due to associated obesity and hyperlipidemia in patients with type 2 diabetes, and the incidence of celiac disease is increased because of a common gene that predisposes to both conditions. Similarly, gastric parietal cell antibodies are found in human leukocyte antigen (HLA) haplotypes that are prevalent in patients with type 1 diabetes (8).
Hormonal Changes in Diabetes
Other hormonal changes described in patients with diabetes play important roles in the pathogenesis of many digestive disorders. For example, increased postprandial hormone release of glucagon and pancreatic peptide in patients with diabetes may exacerbate the disturbed motility in subjects with type 2 diabetes (9). Amylin, a peptide hormone cosecreted with insulin by the pancreatic β-cells, is deficient in patients with type 1 diabetes and elevated in patients in the early stages of type 2 diabetes. Patients in the later stages of type 2 diabetes have reduced amylin secretion that appears before reduced insulin secretion. Amylin and its analogue pramlintide delay gastric emptying (10). Activity of the gastric inhibitory peptide (GIP) that normally inhibits gastric emptying is almost completely lost in patients with type 2 diabetes, although glucagon-like peptide-1 (GLP-1), expressed mainly by the gut L-cells after feeding, maintains its ability to stimulate insulin secretion in these patients, even long after sulfonylurea secondary failure, and may be an important therapeutic alternative in diabetes (11). Ghrelin, a novel peptide hormone synthesized primarily in the stomach, has potent insulin-releasing, appetite-promoting, and gastric promotility effects. This hormone is decreased in the serum of persons with type 2 diabetes (12).
Enteric Neuropathy in Diabetes
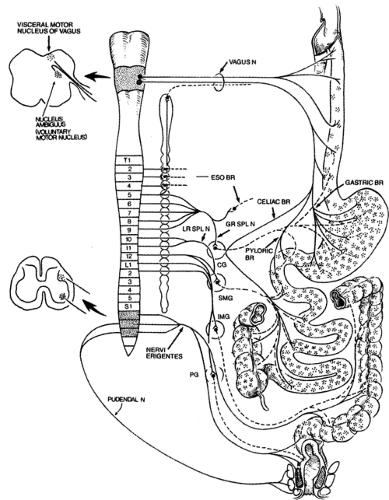
The GI tract is richly innervated by nerves, which can be divided into intrinsic and extrinsic nerves (13). The intrinsic nerves constitute the enteric nervous system (ENS). The extrinsic nerves contain sensory (afferent) and motor (efferent) fibers and are carried along sympathetic, parasympathetic, and somatic pathways to the central nervous system (CNS) (Fig. 64.1). In diabetes, any
one or several of the various components of the nerve elements that control gut function may be involved. The extent of neuropathy appears related to the duration of diabetes (14) and the age of patients (15). Diabetic enteric neuropathy is responsible for many of the GI abnormalities in these patients. The wide spectrum of possible enteric neuropathies may explain the wide range of GI dysfunction in the patient with diabetes.
one or several of the various components of the nerve elements that control gut function may be involved. The extent of neuropathy appears related to the duration of diabetes (14) and the age of patients (15). Diabetic enteric neuropathy is responsible for many of the GI abnormalities in these patients. The wide spectrum of possible enteric neuropathies may explain the wide range of GI dysfunction in the patient with diabetes.
PARASYMPATHETIC INNERVATION
The vagal efferents provide parasympathetic innervation to the entire gut down to the right half of the transverse colon. The left half of the colon and the rectum are innervated by sacral parasympathetic efferents. Parasympathetic preganglionic efferents are thinly myelinated or unmyelinated cholinergic axons. It is now clear that there are two parallel pathway vagal fibers that terminate on excitatory and inhibitory postganglionic neurons, respectively, in the enteric plexuses. The parasympathetic influence on the gut includes precise and localized motor and secretory control activity. The vast majority (>80%) of the vagal fibers are low-threshold afferents that are involved in nonnoxious sensations and primarily mediate reflex activities, including nausea, vomiting, and satiety.
Morphologic studies of vagal abnormalities in diabetes have had variable results. Diani et al. (16) reported marked abnormalities in the vagus nerve in nonketonuric and ketonuric diabetic Chinese hamsters. Analysis of axons from the ventral division of the vagus nerve demonstrated that in the diabetic animals the number of nonmyelinated axons and the numerical and volume density of myelinated fibers were markedly decreased. In an autopsy study of patients with diabetes, Smith (17) demonstrated sparse changes in the vagus nerve. Sections obtained at both the cervical and the diaphragmatic levels of the vagus showed that a small number of fibers had undergone segmental demyelination. A larger number of fibers showed wallerian changes of degeneration. Duchen et al. (18) described more severe pathologic changes of the vagus nerve in an autopsy study of four patients with prolonged diabetes. Guy et al. (19) described pathologic changes in a segment of the abdominal vagus removed during gastric surgery from a patient with severe gastroparesis. These changes included a marked reduction in unmyelinated axons; the remaining axons were characterized by a small diameter with an associated increase in surrounding collagen. In contrast to the above findings, Yoshida et al. (20) found no abnormalities on morphologic analysis of sections of the abdominal vagus nerve in five patients with diabetes, two of whom had symptomatic gastroparesis.
Functional studies provide evidence of parasympathetic efferent denervation of the gut in diabetes. Patients with longstanding diabetes have been found to have an impairment of the cephalic phase of gastric acid secretion. In such patients, sham feeding or insulin-induced hypoglycemia is associated with a diminished secretory response, a finding indicative of decreased vagal influence on the stomach. Moreover, the rise in serum levels of pancreatic polypeptide with sham feeding is also impaired in patients with advanced diabetes, indicating deficient parasympathetic innervation of the pancreas (21).
Of note, vagal or autonomic neuropathy does not uniformly affect innervation to all visceral organs. Similarly, there is no direct correlation between the presence of peripheral neuropathy and autonomic neuropathy. Autonomic neuropathy involving the heart is not predictive of GI dysmotility or of its extent (22).
SYMPATHETIC INNERVATION
The preganglionic neurons of sympathetic efferents are located in the spinal cord (T5 to L3), and the corresponding postganglionic neurons are located in the various sympathetic ganglia. The sympathetic efferent fibers entering the gut are postganglion adrenergic fibers that exert most of their actions indirectly via the enteric neurons. The sympathetic efferents exert inhibitory effects on the gut except in the sphincters, which are contracted by the sympathetic nerves.
In a pathologic study of autopsy findings in diabetic patients, Duchen et al. (18) found several abnormalities in the pattern of sympathetic innervation. In the intermediolateral columns of the spinal cord, where the sympathetic neurons arise, cell numbers appeared reduced at several thoracic levels. In addition, in the cervical and celiac sympathetic ganglia, neurons were distended or vacuolated with enlarged club-shaped neural processes. Chang et al. (23) demonstrated that rats with experimentally induced diabetes have a deficiency in adrenergic-mediated absorption of fluid and electrolyte in the ileum and colon, presumably secondary to deficient sympathetic innervation.
Sympathetic afferents carry visceral nociceptive information to the CNS. They also are involved in many sympathetic reflexes, including nausea and vomiting. It is possible that sympathetic afferent stimulation in neuropathy may be involved in the syndrome of abdominal pain, nausea, and vomiting, and the loss of afferent activity may lead to impaired perception of visceral pain.
ENTERIC NERVOUS SYSTEM
The enteric plexus, which consists of the myenteric and submucous plexuses, forms the ENS, which is the “local brain” of the gut. The ENS resembles the CNS in that it contains sensory, motor, and integrating-command interneurons and program generators. Moreover, ENS neurons, like CNS neurons, employ a large variety of neurotransmitters. These include acetylcholine, neuropeptides such as cholecystokinin (CCK), galanin, calcitonin gene-related peptide (CGRP), gastrin-releasing peptide (GRP), enkephalins, somatostatin, substance P, vasoactive intestinal polypeptide (VIP), purines such as adenosine triphosphate (ATP) and adenosine, and possibly amino acids such as γ-aminobutyric acid (GABA), and nitric oxide (NO). The major inhibitory neurotransmitters are VIP and NO, and the main excitatory neurotransmitters are acetylcholine and substance P.
Previous pathologic studies of diabetic patients in which conventional sections were used found the morphology of the myenteric plexus to be normal. More recent pathologic studies that used tangential sections demonstrated mild abnormalities in these patients. Smith (17) described the myenteric plexus of the esophagus as being mostly normal, with only a small number of neurons with swollen irregular processes. She also described the lymphocytic infiltration of a large number of nonneuronal cells in the plexus. Duchen et al. (18) confirmed this finding in sections from a wider distribution of the gut. Duchen also described infiltration of the ganglia with inflammatory cells, especially around unmyelinated axons. In contrast, Yoshida et al. (20), who prepared extensive sections from the stomachs of patients with diabetes, described the myenteric plexus as being completely normal with no evidence of morphologic change or inflammatory infiltrate.
Rats with streptozotocin-induced diabetes show distinctive and contrasting changes in the nerves in the ileum and proximal colon (24,25,26). In the ileum, immunohistochemical studies show degeneration of adrenergic and serotonin-containing nerves, intact cholinergic nerves, decreased stores of CGRP, increase in VIP and neuropeptide Y, and normal stores of substance P. In contrast, in the proximal colon, the local stores of all these neurotransmitters are either normal or increased. It is noteworthy that the diabetic rats had diarrhea (23). The diminished adrenergic innervation of the ileum might well have contributed to the diarrhea by impairing fluid and electrolyte absorption in this
segment. Mouse models of diabetes also show structural changes in the interstitial cells of Cajal, which are thought to generate electrical pacemaker activity and mediate motor neurotransmission in the stomach. These cells were greatly reduced in the distal stomach, and the normally close associations between these cells and enteric nerve terminals were infrequent in nonobese diabetic (NOD) mice. These observations suggest that damage to interstitial cells of Cajal may play a key role in the pathogenesis of diabetic gastropathy (27).
segment. Mouse models of diabetes also show structural changes in the interstitial cells of Cajal, which are thought to generate electrical pacemaker activity and mediate motor neurotransmission in the stomach. These cells were greatly reduced in the distal stomach, and the normally close associations between these cells and enteric nerve terminals were infrequent in nonobese diabetic (NOD) mice. These observations suggest that damage to interstitial cells of Cajal may play a key role in the pathogenesis of diabetic gastropathy (27).
Functional studies also suggest defects in cholinergic innervation in diabetic rats. Nowak et al. (28) measured the contraction of longitudinal and circular strips of intestine in response to electrical field stimulation in rats with streptozotocin-induced diabetes. Among the three groups of rats, significant differences were seen only in strips of longitudinal muscle from the ileum. The amplitude of contraction was highest in control rats, lowest in diabetic rats, and intermediate in insulin-treated diabetic rats. These changes were seen in the atropine-sensitive contractions, suggesting impaired cholinergic neuromuscular transmission in the distal small bowel in diabetic rats. The relaxatory neuropeptide VIP was found to be increased in immunohistochemical studies of a streptozotocin-treated rat model of diabetes, but electron microscopy revealed degeneration of nerve fibers containing this peptide (29,30). Tissue stores of VIP and its basal release were also decreased in the same diabetic rat model and were partly reversible with insulin therapy (31).
Studies have also shown that nitrergic inhibitory neurotransmission is impaired in diabetics. Decrease in neuronal nitric oxide synthase (nNOS), an enzymatic source of NO, was noted in a number of diabetic animal models, including spontaneously diabetic rats and genetic (nonobese diabetic) and toxin-elicited (streptozotocin) models of diabetes in mice (32,33,34). Watkins et al. (33) demonstrated defects in gastric emptying and nonadrenergic, noncholinergic relaxation of pyloric muscle that resembled defects in mice with a deletion of the nNOS gene. The diabetic mice manifested a pronounced reduction in pyloric nNOS protein and messenger RNA (mRNA). The decline of nNOS in diabetic mice did not result from loss of myenteric neurons. Expression of nNOS and pyloric function were restored to normal levels by insulin treatment. Thus, diabetic gastropathy in mice reflected an insulin-sensitive reversible loss of nNOS. In diabetic animals, delayed gastric emptying could be reversed with a phosphodiesterase inhibitor, sildenafil (33). However, it has also been demonstrated that sildenafil delays gastric emptying of liquids in rats (35). These findings have implications for novel therapeutic approaches and may clarify the etiology of diabetic gastropathy.
SOMATIC INNERVATION
Each end of the GI tube (pharynx, upper esophagus, and external anal sphincter) is composed of striated muscle fibers that are innervated by somatic nerves. Moreover, the parietal peritoneum and abdominal wall receive somatic sensory innervation. Both sensory and motor neuropathies are well-known complications of diabetes. They may cause, on one hand, abnormalities in pharyngeal swallowing and, on the other, external anal sphincter dysfunction during defecation. Sensory neuropathy and radiculopathy may also be responsible for unexplained abdominal pain in patients with diabetes.
The pathogenesis of diabetic neuropathies is not fully understood. Recent revelations of the pathogenesis of diabetic neuropathy may have great bearing on the future prevention of this complication. Biological changes leading to neuropathy that occur with hyperglycemia in patients with diabetes include increased production of advanced glycosylation end products, increased activity of the polyol pathway, disturbance in metabolism of myoinositol and its phospholipid derivatives, elevation of endothelial angiotensin and abnormal permeability of the small blood vessels, impaired neurotrophic support, and impaired resistance to oxidative stress. Currently, treatment of diabetic neuropathy consists of achievement of better glycemic control and treatment of symptoms related to neuropathy. Specific treatments capable of preventing or curing neuropathy are being studied. With the introduction of potent aldose reductase inhibitors, the role of increased activity of the polyol pathway (and related abnormalities in myoinositol metabolism) in the pathogenesis of diabetes-associated complications may be clarified. Despite interesting results obtained with aldose reductase inhibitors in animal studies, initial results in patients with diabetes are less encouraging (36). Other metabolic approaches, such as antioxidants and γ-linolenic acid supplementation, seem promising (37). Clearly, early detection of diabetic neuropathy is required, because at present a preventive approach is the most effective way to avoid or postpone debilitating complications. More research is needed to make effective curative treatments of diabetic neuropathy available.
Gut Smooth Muscle in Diabetes
In general, the intestinal smooth muscle in diabetes is normal and functionally intact. Although the primary disorder of gut motility appears to be one of hypomotility or even atony, experimental observations strongly argue that these changes are the result of deficient innervation. When cholinergic agonists are administered, contractions are of normal amplitude (38). In an extensive review of many sections obtained from all parts of the stomach, Yoshida et al. (20) demonstrated normal smooth muscle without evidence of degeneration or vacuolation. In contrast, Duchen et al. (18) and Guy et al. (19), in a small number of patients, described morphologic abnormality of intestinal smooth muscle. Both groups of investigators observed eosinophilic or hyaline-like bodies (rounded or club shaped) lying in or replacing smooth muscle cells. The extensive autopsy studies of Duchen et al. (18) described the presence of these smooth muscle bodies throughout the GI tract and, in addition, in the smooth muscle of the bladder. The significance of these hyaline bodies is unclear, but the preponderance of clinical data indicates that the intestinal smooth muscle is functionally healthy. Histologic changes in gastric smooth muscle were also reported in overt gastroparetic patients with longstanding type 1 diabetes, including smooth muscle degeneration and fibrosis, with eosinophilic inclusion bodies (M-bodies), which appear to be unique to this condition (39).
There are also electrophysiologic changes in the gut smooth muscle with diabetes. In rat models of type 2 diabetes, for example, the gastric fundus shows functional impairment of neuromuscular transmission, reduced maximum activity of the electrogenic pump, increased sensitivity of muscarinic receptors, reduced sensitivity of adrenoreceptors, and reduced myogenic activity in gastric smooth muscles. These alterations in the properties of smooth muscle may be involved in diabetes-induced gastroparesis (40). The colonic smooth muscle also shows changes, including a more depolarized membrane and a reduction in reactivity of adrenoreceptors to noradrenaline. However, there is also notable attenuation of nonadrenergic noncholinergic inhibitory transmission, suggesting that the constipation appearing with diabetes involves dysfunction of both the enteric autonomic nerves and the smooth muscles in the colon (41).
Microangiopathic Changes in Diabetes
Microangiopathic changes in the GI tract of patients with diabetes are frequently mentioned in the clinical literature. De Las Casas and Finley (42) reported pathologic studies documenting
these changes in duodenal biopsies from a patient with longstanding type 1 diabetes and chronic diarrhea. They described striking histopathologic findings of diabetic microangiopathy, including prominent mural thickening and luminal narrowing of blood vessels within the duodenum secondary to accumulation of hyaline material, which was periodic acid-Schiff positive and intensely stained with monoclonal antibodies to type IV collagen. Potential mechanisms for diabetes-specific microvasculature disease, including decreased vasodilators such as NO, increased vasoconstrictors such as angiotensin II and endothelin-1, and increased permeability factors such as vascular endothelial growth factor have recently been reviewed (43).
these changes in duodenal biopsies from a patient with longstanding type 1 diabetes and chronic diarrhea. They described striking histopathologic findings of diabetic microangiopathy, including prominent mural thickening and luminal narrowing of blood vessels within the duodenum secondary to accumulation of hyaline material, which was periodic acid-Schiff positive and intensely stained with monoclonal antibodies to type IV collagen. Potential mechanisms for diabetes-specific microvasculature disease, including decreased vasodilators such as NO, increased vasoconstrictors such as angiotensin II and endothelin-1, and increased permeability factors such as vascular endothelial growth factor have recently been reviewed (43).
ABDOMINAL PAIN IN DIABETES
Both acute and chronic abdominal pain can present rather uniquely in patients with diabetes. Syndromes of acute and chronic abdominal pain can masquerade as disorders of intraabdominal or pelvic pathology and must be recognized to permit the institution of appropriate therapy.
Acute Abdominal Pain
Acute abdominal pain, tenderness, and vomiting have long been recognized as frequent in patients presenting with diabetic ketoacidosis (44). Unexplained abdominal pain in the setting of diabetic metabolic decompensation tends to be generalized or epigastric in location. The mechanism of acute abdominal pain is not clear. Hyperamylasemia can occur but is not correlated with the presence of pancreatitis. It has been suggested that abdominal pain and vomiting might be due to the gastric dilatation and intestinal ileus that can occur secondary to the metabolic acidosis. Theoretically, the pain could be due to a stretching of the hepatic capsule in response to hepatic steatosis; abrupt hepatic distention, however, is unlikely to be due to steatosis. Finally, acute pain may simply be due to activation of nociceptors in response to metabolic derangements.
Campbell et al. (44) reviewed the clinical findings and outcome in 211 episodes of metabolic decompensation in 140 patients with diabetes over an 8-year period. Forty-four patients experienced severe abdominal pain and tenderness that necessitated diagnostic evaluation. In 17 patients, the abdominal pain could be attributed to an underlying disorder (e.g., pyelonephritis or appendicitis) considered to have precipitated the metabolic decompensation. In the other 29 patients, the abdominal pain remained unexplained and was attributed to the ongoing ketoacidosis. Patients with unexplained abdominal pain were younger than 40 years of age and, with only three exceptions, had a plasma bicarbonate level of less than 10 mEq/L. The authors suggested that acute abdominal pain in patients with diabetes older than 40 years of age or with plasma bicarbonate levels greater than 10 mEq/L should not be attributed to the metabolic decompensation and that a search should be undertaken for an underlying abdominal or pelvic disorder. In all patients, GI, renal, and pelvic diseases should be excluded, especially in those with fever, localized abdominal pain or tenderness, or abnormal laboratory findings. The acute abdominal pain associated with diabetic ketoacidosis resolves with correction of the metabolic abnormalities. It is important to recognize this entity to avoid unnecessary and harmful exploration laparotomy in these patients.
Chronic Abdominal Pain
Chronic abdominal pain can occur as a result of diabetic sensory neuropathy and can masquerade as serious intraabdominal pathology, especially when it is associated with weight loss. Thoracic polyradiculopathy is an important cause of chronic pain in patients with diabetes. Longstreth (45) described the syndrome of chronic abdominal pain and weight loss caused by thoracic radiculopathy in 10 middle-aged or elderly patients with type 2 diabetes. Some of these patients initially underwent investigations focused on possible malignancy. Some had even undergone laparotomy in a search for possible carcinoma of the pancreas. In the affected patients, the pain tended to be asymmetric rather than bilateral and most often affected the left upper abdomen and often radiated into or from the lower thoracic spine. At times both the upper abdomen and the lower chest were involved in the pattern of symptoms. The pain was described as a pressure discomfort or sharp pain and at times had neuropathic qualities such as “burning” or “stabbing.” The pain was often worse at night and aggravated by light pressure. The onset of the pain was gradual, being at first intermittent, later more frequent, and finally constant. It is especially noteworthy that the pain was not brought on or affected by either eating or defecation, a possible clue that the pain did not originate from the GI tract. Marked weight loss—up to 19 kg—occurred in some of the patients and presumably was caused by pain-induced anorexia. The diagnosis of thoracic radiculopathy secondary to diabetes was confirmed by electromyographic demonstration of either unilateral or bilateral denervation of the paraspinal muscles in the middle thoracic to upper lumbar region in seven of the patients. Nine patients recovered spontaneously, but two had recurrent polyradiculopathy. A combination of nonsteroidal antiinflammatory drugs and tricyclic antidepressants has been used in other syndromes of radiculopathy and/or peripheral neuropathy and is worth a trial in this syndrome.
PHARYNX AND ESOPHAGUS IN DIABETES
Pharyngeal and esophageal motor abnormalities are frequently found in persons with diabetes and are more prevalent in patients with peripheral or autonomic neuropathy. However, these motor abnormalities rarely produce significant symptoms. Thus, dysphagia and chest pain should be thoroughly evaluated and not ascribed to the diabetes. The incidence of reflux esophagitis and candida esophagitis may be increased in patients with diabetes.
Pharyngeal Motility
In barium studies, Borgstrom et al. (46) evaluated “swallowing complaints” in 18 patients with diabetes, 16 of whom had evidence of autonomic neuropathy. Videofluoroscopy of the pharynx demonstrated motor abnormalities in 14 patients. These included defective epiglottic mobility, defective closure of the laryngeal vestibule, and weakness of the pharyngeal musculature. When symptoms of pharyngeal dysphagia are present, swallowing therapy may be helpful in the management of these patients.
Esophageal Motility
Several prospective studies have demonstrated that abnormalities of esophageal motility and transit are quite common among diabetic patients with neuropathy but are usually asymptomatic. Horowitz et al. (47) performed scintigraphic studies of esophageal emptying of a solid bolus and found delayed emptying in 42% of patients with type 1 diabetes and in 30% of patients with type 2 diabetes who were receiving oral hypoglycemic agents. Other groups have reported similar results (48,49).
Manometric studies of esophageal motility are an even more sensitive measure of esophageal motor dysfunction. Hollis et al. (50) studied esophageal motility in patients with diabetes and found that 56% had abnormal esophageal motility. Abnormalities were more common in patients with diabetic neuropathy. Among those with evidence of peripheral sensory neuropathy but not of autonomic neuropathy, 80% had abnormalities in esophageal motility. All four patients in this study who had evidence of autonomic neuropathy showed abnormalities of esophageal motility. In patients with peripheral neuropathy alone, esophageal motility showed (a) contractions of low or normal amplitude, (b) some decrease in velocity of peristalsis, (c) an increased frequency of dropped swallows, and (d) a mild delay of transit. Nevertheless, in patients with peripheral neuropathy alone, the majority of swallows are associated with normal peristalsis. Diabetic patients with autonomic neuropathy have an increased frequency of multipeaked and simultaneous contractions. Huppe et al. (51) and Loo et al. (52) reported similar abnormalities. Correlating scintigraphic studies of esophageal transit to manometric studies, Keshavarzian et al. (53) found that multipeaked contractions usually are associated with normal esophageal transit but that simultaneous contractions are associated with delayed transit. Another group showed that, in type 1 diabetes, retarded esophageal transit usually reflects either peristaltic failure or focal low-amplitude pressure. In these studies, as in others, the overwhelming majority of patients were free of esophageal symptoms. Even patients with motility patterns compatible with diffuse esophageal spasm were usually asymptomatic. Chest pain was a rare symptom (54).
The mechanism of the esophageal motor abnormalities in diabetes remains unclear. Loo et al. (52) reported that the administration of atropine inhibited the development of the second esophageal peak. It has been suggested that the presumed loss of vagal innervation of the esophagus is the mechanism of abnormal peristalsis, although experimental demonstration of this theory has not been documented. In one study, disorder of motor nerve conduction velocity correlated with esophageal motility disorders, but no significant correlation could be found between esophageal dysfunction and diabetic autonomic neuropathy, as measured by coefficient of variation of cardiac R-R intervals (55).
Clouse et al. (56) suggested that the esophageal motor abnormalities observed in patients with diabetes were due to coincident psychiatric disease (depression and anxiety disorders) rather than to neuropathy. Psychiatric illness, as defined by testing, was present in 87% of those diabetic patients with motor abnormalities but in only 21% of those with normal motility. This association was independent of neuropathy. In this study, the vast majority of patients were without esophageal symptoms.
Reflux Esophagitis
In patients without esophageal symptoms, abnormal gastroesophageal reflux on ambulatory pH testing was significantly more prevalent in insulin-dependent diabetics (28% more) compared with healthy persons without diabetes. Moreover, reflux was associated with cardiovascular autonomic neuropathy (abnormal reflux = 38.7% in diabetic patients with cardiovascular autonomic neuropathy and 10.5% in diabetic patients without autonomic neuropathy) (57). In patients with symptomatic diabetic gastroparesis, the combination of gastric distention and increased gastric residual volume may make gastroesophageal reflux a more common occurrence. Jackson et al. (58) compared 24-hour esophageal pH, autonomic function testing, and electrogastrography (EGG) in two patient groups with symptoms of gastroesophageal reflux disease: one group with diabetes and one without diabetes. In this study, those with diabetes frequently had normal 24-hour pH but abnormal autonomic functioning. In contrast, those without diabetes had abnormal 24-hour pH but normal autonomic function. The two groups had identically abnormal mean EGG values. In patients with diabetes, the lower esophageal sphincter generally demonstrates normal pressures and relaxation (50). Therefore, it is unclear whether gastroesophageal reflux is more frequent in diabetic individuals than in normal controls, although the symptom of heartburn is less frequent in the patients with diabetes (7). The decreased symptoms of heartburn may be related to sensory impairment in these patients. The treatment of reflux esophagitis in patients with diabetes is no different from that in patients without diabetes (4).
Candida Esophagitis
An important esophageal complication of diabetes is candida esophagitis. Impaired immunity associated with diabetes is believed to increase susceptibility to this disorder. Moreover, stasis of esophageal contents associated with abnormal motor function could contribute to the susceptibility to candida infection. Extensive esophageal candidiasis may be asymptomatic or may be associated with symptoms of odynophagia (pain on swallowing) or dysphagia. Presence or absence of oral candidiasis has no reliable predictive value for esophageal candidiasis. The technique of barium swallow is relatively insensitive for detecting this disorder; with advanced esophageal candidiasis, a single- or double-contrast barium swallow may demonstrate extensive mucosal abnormalities suggestive of esophagitis. Fiberoptic esophagoscopy is much more sensitive and often demonstrates innumerable scattered white plaques over variable lengths of the esophagus. Esophageal brushings obtained through the endoscope can confirm the diagnosis.
Therapy for affected diabetic persons with otherwise intact immunity most commonly consists of nystatin suspension, 1 to 3 million units administered orally four times per day, or 10 mg of clotrimazole troches given orally five times per day, which are equally effective. More effective is fluconazole, 200 mg on day 1, followed by 100 mg once a day for a minimum of 3 weeks and at least 2 weeks after symptom resolution to prevent recurrence. If the patient is unresponsive, swish and swallow of itraconazole solution, 200 mg per day, for 2 weeks after symptom resolution, for a total of 3 weeks, is often prescribed. Ketoconazole may be somewhat less effective but is only one-third the cost of itraconazole. However, potential for hepatotoxicity has been recognized. The absorption of either itraconazole or ketoconazole is significantly decreased with antacids, and the two should not be administered concomitantly. Relapse is common and often occurs 2 to 4 months after clinical cure. Refractory esophageal candidiasis due to drug resistance may require systemic therapy with amphotericin B. Newer antifungal drugs being developed for fluconazole-resistant Candida include rilopirox (59) and voriconazole (60). Immunologic treatments being developed include vaccines and antibodies to Candida.
Dysphagia in Diabetes
Although minor pharyngeal and esophageal motor abnormalities are quite common in diabetes, they are usually asymptomatic (46). Because this is a disorder associated with few, if any, symptoms, specific therapy is generally not needed. In general, prokinetic agents (metoclopramide or domperidone), which have been shown to enhance esophageal contractions, have not been shown to improve esophageal transit in patients with delayed transit, as demonstrated by scintigraphic techniques. In one study, acute administration of oral cisapride improved
esophageal transit in patients with baseline slowing of transit (61). Orally administered erythromycin has also been shown to improve esophageal transit after 2 weeks of therapy (62). After 1 year of treatment with tolrestat, an aldose-reductase inhibitor, patients with type 2 diabetes with asymptomatic diabetic neuropathy showed significant improvement in esophageal transit time on scintigraphy (63).
esophageal transit in patients with baseline slowing of transit (61). Orally administered erythromycin has also been shown to improve esophageal transit after 2 weeks of therapy (62). After 1 year of treatment with tolrestat, an aldose-reductase inhibitor, patients with type 2 diabetes with asymptomatic diabetic neuropathy showed significant improvement in esophageal transit time on scintigraphy (63).
It is not known if the relative lack of esophageal symptoms in patients with diabetes is due to changes in sensory threshold caused by associated sensory neuropathy involving the esophagus. Feldman and Schiller (3) reported that 27% of unselected patients with diabetes complained of some dysphagia. In general, significant dysphagia in a patient with diabetes should not be explained by the diabetic pharyngeal or esophageal motility abnormalities, and a search for another associated cause of dysphagia should be undertaken.
STOMACH IN DIABETES
Gastric Motor Activity
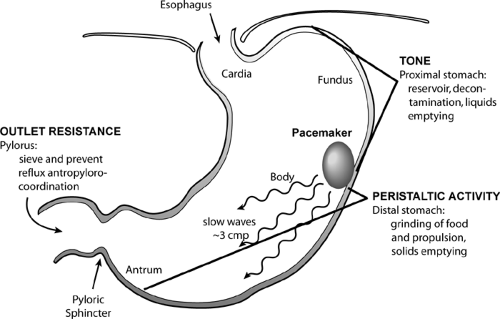
The stomach normally performs four distinct functional motor activities. It (a) acts as a reservoir to accommodate the volume of solids and liquids of a meal; (b) pulverizes solids and mixes them with gastric acid to reduce the particle size for optimal digestion; (c) empties liquids and pulverized solids into the duodenum during the postprandial digestive period; and (d) empties the remaining food residues, including indigestible material, during the interdigestive period. These actions are achieved in different functional compartments of the stomach, i.e., the proximal stomach, the distal stomach, and the pyloric sphincter region (Fig. 64.2). In diabetes, all of these functions may be impaired (Table 64.1).
TABLE 64.1. Gastric Motor Abnormalities in Diabetes Mellitus | ||||||
|---|---|---|---|---|---|---|
|
PROXIMAL STOMACH
The proximal stomach consists of the fundus and the orad third of the gastric body. The proximal stomach exhibits tonic contractions, producing prolonged elevations in pressure lasting 1 to 6 minutes. These contractions press gastric contents aborally toward the distal stomach and the duodenum and play an important role in gastric emptying. They are stimulated by excitatory fibers in the vagus and by hormones such as motilin. Normally, the proximal stomach relaxes with each swallow (“receptive relaxation”) and also as the volume of swallowed food builds up (“accommodation”). With receptive relaxation and accommodation, the stomach can hold increasing volumes without increasing gastric pressure, which enhances its function as a reservoir. Receptive relaxation is mediated by inhibitory fibers in the vagus nerve, whereas accommodation is mediated by inhibitory neurohormonal influences. The resting tone of the fundus in patients with diabetes is comparable to that in healthy subjects, but fundic contractions, as measured by a motility index, are reduced in patients with diabetes (64). The status of the receptive relaxation or accommodation in patients with diabetes is poorly characterized, but these inhibitory responses are thought to be impaired owing to the loss of inhibitory nerves in patients with diabetes.
DISTAL STOMACH
In the distal two thirds of the stomach, contraction waves arise proximally and distally to the gastroduodenal junction, carrying part of the gastric contents ahead of the wave. In the interdigestive (fasting) period, four phases of variable motor activity occur in a cyclic fashion (65). During phase I, few, if any, contractions occur in the stomach. During phase II, intermittent random contractions are propagated distally over short distances. This is followed by phase III, which is a brief complex of rhythmic (three cycles per minute), strong propulsive contractions. Phase III originates in the proximal stomach and migrates through the distal stomach. This succession of cycles has been termed the interdigestive migrating motor complex (IMMC), and the period of an entire cycle is approximately 100 minutes. In the digestive (postmeal) period, the phase III activity, if present, is abolished and replaced by the phase II-like activity. The rate of contractions in the distal stomach always occurs at a predictable time that is a multiple of three or four, such that maximal rate of the phasic contractions in the distal stomach is never more than three or four per minute. This rhythm of the peristaltic contractions is paced by the underlying intrinsic electrical waves (called slow waves) that arise from pacemaker cells identified as the interstitial cells of Cajal. These slow waves occur at a rate of about three or four cycles per minute. These cycles originate in a region of the greater curvature of the gastric body (gastric pacemaker) and move distally toward the pylorus at an accelerating rate of 0.5 to 4 cm per second.
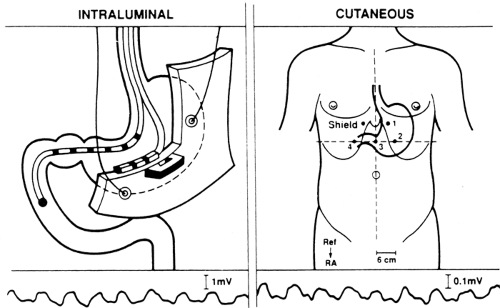
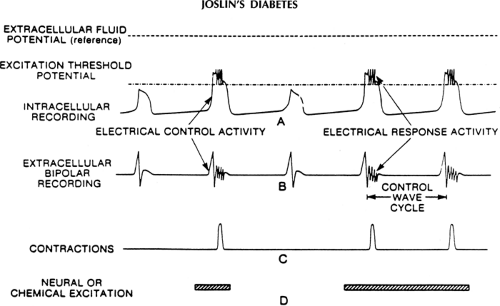
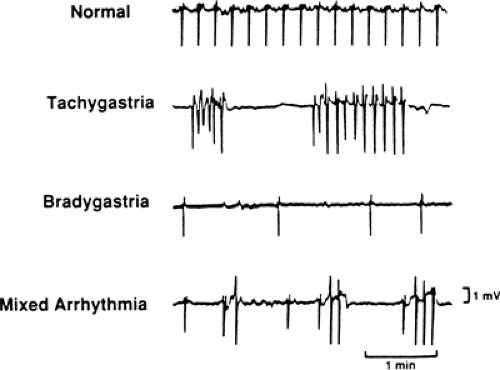
Gastric slow waves can be recorded by surface electrodes; this recording is called an electrogastrogram (EGG, Fig. 64.3). When the depolarizations become large, they are superimposed by action potentials, causing gastric contractions (Fig. 64.4). On the other hand, when the amplitude of slow-wave depolarizations decreases, no action potentials are triggered and no contractions occur. The slow-wave rhythm also influences the strength of their depolarizations. The decreased amplitude of depolarization results from abnormal rhythms (dysrhythmia) of gastric slow waves such as tachygastria (increased slow-wave frequency), bradygastria (decreased slow-wave frequency), or gastric arrhythmia (irregular slow-wave frequencies) (Fig. 64.5) (66,67). Gastric slow-wave frequencies and amplitude of depolarizations (also described as power of the slow waves) can be monitored with cutaneous EGGs.
Some patients with diabetic gastroparesis have gastric dysrhythmia. In one study, nine of ten patients with diabetic gastroparesis had runs of tachygastria as compared with only one subject from a comparable control group (67). Similarly, in another study of six patients with diabetic gastroparesis, one had tachygastria, two had bradygastria, and the remaining three had flat-line patterns on cutaneous gastrograms (68). In yet another study, patients with diabetic gastroparesis had normal slow-wave cycles but lacked the normal postprandial increase in strength of the slow waves (9). Such electrogastrographic abnormalities could also be demonstrated in a high proportion of children with insulin-dependent diabetes, which correlated with both poor hyperglycemic control and delayed gastric emptying (69). Patients with diabetic gastroparesis may show decreased or normal cycle numbers, decreased or normal amplitudes, and peristaltic or nonperistaltic contractions in any permutation and combination (70,71). The motility index, which is the product of amplitude and number of contractions over time, does not take into account the peristaltic behavior of the contractions. Therefore, the clinical importance of changes in the motility index in diabetes is limited. Fischer et al. (9) observed that the normal postprandial increase in antral motility and myoelectrical activity was missing in patients with diabetes with symptomatic gastroparesis. Malagelada et al. (72) demonstrated that patients with diabetes with symptomatic gastroparesis had no antral IMMCs but had normal IMMCs in the duodenum. In patients with diabetes without symptoms of gastroparesis, IMMC activity was present in both the antrum and the duodenum, whereas symptomatic patients with diabetic gastroparesis had no IMMC activity in the antrum and, for some, in the duodenum (73). In contrast to the general pattern of decreased gastric motor activity, occasional patients with diabetes demonstrate fasting patterns of ectopic and aberrant antral motility. In some patients, episodes of sustained high-frequency activity (three cycles per minute) that is neither propagated nor associated with IMMC activity have been observed in the antrum. These motor abnormalities may also cause gastric stasis. This phenomenon has been related to intestinal motor aberrations created by sympathetic denervation (74).
In diabetes, changes in gastric slow waves and contractions may be due to several factors, including changes in innervation and metabolic abnormalities. Vagal innervation plays a very
important role in determining the rate, rhythm, and propagation of slow waves and, therefore, in the number, rate, and peristaltic behavior of contractions. Vagal innervation of the stomach is essential for interdigestive cyclic motor activity. With vagotomy, fasting motor activity in the stomach is abolished and the IMMC originates in the duodenum distal to the stomach (65). The cyclic release of motilin from endocrine cells in the duodenum and jejunum plays an important role in initiating IMMCs in the stomach. This cyclic motilin release is mediated by vagal cholinergic influences in the dog but perhaps not in humans. Fox and Behar (38) suggested that this decreased activity in the distal stomach is due to decreased cholinergic transmission; when such patients were treated with parenteral bethanechol, the amplitude and frequency of contractions increased to normal levels.
important role in determining the rate, rhythm, and propagation of slow waves and, therefore, in the number, rate, and peristaltic behavior of contractions. Vagal innervation of the stomach is essential for interdigestive cyclic motor activity. With vagotomy, fasting motor activity in the stomach is abolished and the IMMC originates in the duodenum distal to the stomach (65). The cyclic release of motilin from endocrine cells in the duodenum and jejunum plays an important role in initiating IMMCs in the stomach. This cyclic motilin release is mediated by vagal cholinergic influences in the dog but perhaps not in humans. Fox and Behar (38) suggested that this decreased activity in the distal stomach is due to decreased cholinergic transmission; when such patients were treated with parenteral bethanechol, the amplitude and frequency of contractions increased to normal levels.
Acute changes in blood glucose concentration can have major effects on GI motor function. Barnett and Owyang (75) demonstrated that experimentally induced hyperglycemia in normal subjects reduced antral motor activity and abolished the gastric component of the IMMC. With increasing hyperglycemia, levels of serum motilin fell. Because motilin is postulated to be a physiologic regulator of gastric IMMC activity, the authors suggested that the hyperglycemic reduction in motor activity might be due in part to a hyperglycemic reduction in the levels of serum motilin. The investigators noted, however, that gastric IMMC activity was reduced at levels of blood glucose that had no effect on the serum motilin level. Hence they
suggested that hyperglycemia per se might also decrease antral motor activity independent of changes in serum motilin levels. Serum motilin levels were also elevated in patients with diabetes, suggesting therefore that the absence of gastric IMMC activity in these patients was not due to a deficiency of circulating serum motilin (75,76). The group also showed that hyperglycemia causes gastric slow-wave dysrhythmias that could be prevented by administration of indomethacin in healthy volunteers (77). Subsequently, others have shown that hyperglycemia attenuates the stimulation of antral pressures and propagates antral sequences by the motilin receptor agonist erythromycin (78) and attenuates the expected acceleration of both solid and liquid emptying with erythromycin treatment (79,80). Yet others have implicated the role of dopamine stimulation in hyperglycemic disturbance of gastric motility (81,82).
suggested that hyperglycemia per se might also decrease antral motor activity independent of changes in serum motilin levels. Serum motilin levels were also elevated in patients with diabetes, suggesting therefore that the absence of gastric IMMC activity in these patients was not due to a deficiency of circulating serum motilin (75,76). The group also showed that hyperglycemia causes gastric slow-wave dysrhythmias that could be prevented by administration of indomethacin in healthy volunteers (77). Subsequently, others have shown that hyperglycemia attenuates the stimulation of antral pressures and propagates antral sequences by the motilin receptor agonist erythromycin (78) and attenuates the expected acceleration of both solid and liquid emptying with erythromycin treatment (79,80). Yet others have implicated the role of dopamine stimulation in hyperglycemic disturbance of gastric motility (81,82).
Regardless of the motility changes ascribed to hyperglycemia, the impact of chronically elevated blood glucose concentrations on the rate of gastric emptying remains unclear. An inverse relationship between rate of gastric emptying and blood glucose levels has been reported: Gastric emptying is slower during hyperglycemia and faster during hypoglycemia (83). However, there is also contrary evidence for delayed gastric emptying with hypoglycemia (84). Moreover, Holzapfel et al. (85) reported that there was no relation between emptying and fasting blood glucose concentration, its postprandial increase, or their reduction to euglycemic values in patients with type 2 diabetes. These data did not support a major role of hyperglycemia in gastric stasis.
Fischer et al. (9) proposed that higher-than-normal postprandial blood levels of glucagon may, at least in part, be responsible for disturbed gastric motility in patients with type 2 diabetes. Frank et al. (86) indeed found higher postprandial levels of glucagon and lower insulin concentrations in the nonneuropathic patients with type 2 diabetes in their study. Meanwhile, entry of ingested glucose into the blood and the levels of other various enteropeptides (CCK, glucose-dependent insulinotropic polypeptide, neurotensin, and peptide YY) were similar to that in subjects without diabetes, suggesting that the postprandial hyperglycemia was from hepatic release.
PYLORUS
The gastric pylorus is a narrow channel that can actively change the size of its opening under the influence of excitatory and inhibitory nerves. The pylorus is not a usual sphincter, because under basal conditions its resting pressure is not elevated (65). The opening size of the pylorus determines not only the rate of gastric emptying but also the size of the food particles that are permitted to leave the stomach. Soon after a meal, as the peristaltic waves in the stomach and antrum carry pieces of food toward it, the pylorus opens partially so that only liquids or small particles pass through, and solid chunks of food are trapped in the antrum to be ground by powerful antral contractions. If the pylorus does not open or relax, gastric emptying of liquids, as well as of ground and unground solids, is inhibited and gastric stasis occurs. It has been reported that luminal contents in the small bowel may inhibit gastric emptying by enhancing pyloric closure via neurohormonal reflexes (65). Wider opening of the pylorus is also essential for movement of large pieces of food during the IMMC.
Careful manometric studies have shown that patients with diabetes have increased fasting and postprandial pyloric motor activity. In addition, these patients demonstrate episodes of “pylorospasm” characterized by prolonged periods of increased tonic and phasic motor activity in this region (87). It has been suggested that pyloric motor activity and pylorospasm could act as a “brake” on gastric emptying and hence could contribute to the morbidity and disability of gastroparesis. The increased pyloric motor activity might be due to an increase in cholinergic or noncholinergic excitatory nerve activity or to a decrease in adrenergic or nonadrenergic (VIPergic and nitrergic) activity with resultant decreased pyloric inhibition.
DUODENUM
Normally, duodenal activity is coordinated with antral and pyloric activity. During the period of enhanced gastric emptying, the duodenal and pyloric activities are inhibited with each antral peristalsis. The inhibition is followed by contractions that form a peristaltic sequence with antral contractions. Such a duodenal inhibition can be called receptive relaxation of the duodenum and is due to inhibitory neural influences. Impairment of this duodenal relaxation results in antroduodenal incoordination, and this acts to inhibit gastric emptying. However, the importance of antroduodenal incoordination in diabetic gastroparesis is unclear. Duodenal mucosal afferent nerves also play an important role in reflex modulation of gastric emptying of liquids based on the composition of a liquid meal emptied from the stomach (65). Liquid meals of high caloric densities, high fat content, high osmolality, and acid pH stimulate duodenal receptors to inhibit gastric emptying. No information is available on duodenogastric reflexes in diabetic gastroparesis.
Gastric Emptying
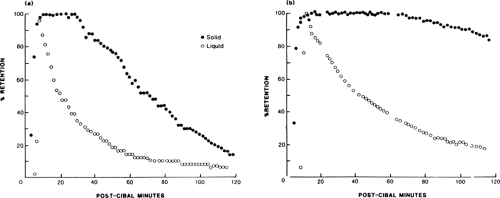
The motor activities of various parts of the stomach are well designed to regulate the emptying of different physical constituents of food, so that the liquid and digestible solid components are emptied in the digestive period (within 2 to 3 hours after ingestion) and indigestible solids are emptied from the stomach during the interdigestive period (2 to 3 hours after a meal). In diabetic gastroparesis, gastric emptying of all these components is affected to varying degrees, depending upon the stage of the disease and the underlying pathophysiologic defects. Figure 64.6 shows patterns of gastric emptying of liquids and solids in one patient with diabetic gastroparesis and in a normal control.
EMPTYING OF LIQUIDS
Gastric emptying of liquids is normally influenced by the composition of the liquid meal, whose characteristics—e.g., acidity, caloric and nutritional content, and osmolality—elicit different responses from duodenal receptors and, in turn, from reflexes responsible for emptying (65). Hence, the liquid emptying varies in different studies because of the use of meals ranging from simple solutions (water, 10% dextrose, and orange juice) to complex nutritional solutions that truly justify the term “liquid meal.” However, even with a standardized meal, gastric emptying of liquids in patients with diabetes is variable. Keshavarzian and Iber (88) demonstrated rapid emptying of liquids in patients with diabetes and suggested that it was due to diminished receptive relaxation in the diabetic stomach. In contrast, Loo et al. (89) and Wright et al. (90) demonstrated normal emptying of liquids in patients with diabetes. Others have demonstrated delayed emptying of liquids in patients with diabetes (91,92,93). The delayed emptying of liquids may be due to reduced fundic motor activity and antral motility. Another factor that could affect liquid gastric emptying is hyperglycemia. Induced hyperglycemia in normal subjects is associated with a slowing of the gastric emptying of liquid meals containing fat and protein; hyperglycemia may cause a reflex decrease in vagal excitatory tone of the stomach with a resulting delay in gastric emptying (94). There was no correlation between the degree of delay of emptying of liquids and the delay in emptying of solids (91).
EMPTYING OF DIGESTIBLE SOLIDS
Normally, in the postprandial period, digestible solids are emptied more slowly than liquids because solids must be pulverized to a size (<2 mm) sufficient to pass through the sieve created by the contracted pyloric sphincter. In patients with diabetes, the gastric emptying of solids is frequently delayed. Horowitz et al. (47) studied gastric emptying among unselected patients with diabetes. Emptying of solids was delayed among 58% of patients with type 1 diabetes (61) and 30% of patients with type 2 diabetes.
EMPTYING OF INDIGESTIBLE SOLIDS
Indigestible solids, such as dietary fiber, normally do not empty during the postprandial period of motor activity because of the functional sieving produced by the pyloric sphincter (65). Such ingredients of food are emptied during the interdigestive period by phase III activity of the IMMC. Since gastric IMMC activity is impaired and often absent in symptomatic patients with diabetes, the emptying of indigestible solids from the stomach is delayed in these patients.
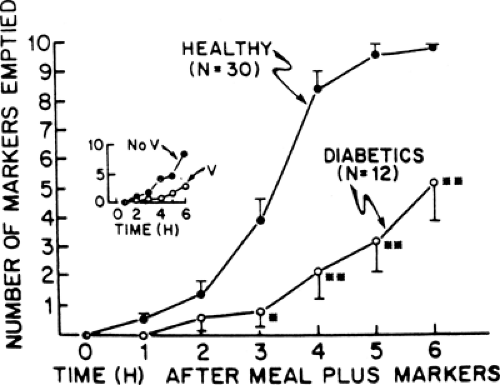
Feldman et al. (95) studied the emptying of indigestible solids in normal and diabetic subjects. Normal subjects emptied all of the ingested markers within 6 hours, a rate faster than that of the patients with diabetes (Fig. 64.7). A gastric-emptying study using a radiopaque marker identified patients with diabetes with symptoms of gastroparesis more accurately than did a scintigraphic technique using a meal of 99Tc-labeled scrambled eggs. Similar radiopaque markers have been validated by other groups in the diagnosis of diabetic gastroparesis (96,97).
Radiopaque-marker gastric-emptying studies measure both lag time at which the interdigestive motor pattern reappears after a meal and the efficacy with which phase III motor activity empties the indigestible markers. Patients who lack IMMC activity in the stomach and have delayed gastric emptying of radiopaque markers are more likely to have abnormal gastric emptying of dietary fiber and would be more susceptible to the formation of gastric bezoars. If the pyloric sphincter fails to open widely because of either a motility abnormality or partial mechanical stenosis, gastric stasis of indigestible food would occur. However, a recent study cast doubt on the relationship of antral phase III activity in emptying indigestible markers as established from animal studies. Radiopaque markers (1.5-mm, 3-mm, and 7-mm cubes) were given with a test meal and followed by fluoroscopy with simultaneous antral manometry in normal subjects. None of the subjects had an antral phase III before all markers were emptied from the stomach. Instead, the typical irregular postprandial pressure activity was present in all subjects until the emptying was completed, and the highest postprandial motility index during the emptying study was comparable to the motility index during late phase II. Contrary to common opinion, the occurrence of gastric emptying of indigestible solids after a meal can be unrelated to the antral phase III, at least up to a particle size of 3 mm and perhaps even 7 mm (98).
Diabetic Gastropathy
Diabetic gastropathy is defined as a symptom complex with functional, contractile, electrical, and sensory dysfunction of the stomach associated with diabetes. In its classical form, called diabetic gastroparesis, it is associated with delayed gastric emptying. However, many patients with dyspeptic symptoms have normal or even enhanced gastric emptying. Thus even the definition of what constitutes a clinically relevant abnormality of gastric motility remains unclear.
DIABETIC GASTROPARESIS
Delayed emptying of solid or nutrient meals is found in up to 50% of patients with type 1 diabetes and in 30% of patients with type 2 diabetes (94). However, the degree of delay for various constituents of food, i.e., liquids, digestible solids, and indigestible solids, is not the same. Simultaneous assessment of gastric emptying of liquids and digestible solids using dual markers (99Tc-labeled solid phase and 111In-labeled liquid phase) showed that indigestible solids are particularly delayed in persons with diabetes (96,99). Figure. 64.6 shows patterns of gastric emptying of liquids and solids in one patient with diabetic gastroparesis and in a healthy control.
There is a wide range of symptoms in diabetic gastroparesis, and the degree of delayed gastric emptying correlates poorly with severity of symptoms. Many patients with abnormal gastric emptying have no specific symptoms and may be found to have a gastric bezoar or a largely dilated stomach with retained contents. Frequently, however, these patients have symptoms of anorexia, early satiety, and postprandial abdominal fullness and discomfort that resemble simple dyspepsia. Vomiting of old food, however, is indicative of gastroparesis. Nausea and vomiting are common when gastric distention is associated with obstruction and vigorous gastric contractions. In some patients, atonic dilation of the stomach, even when massive, may not be associated with nausea or vomiting. Nausea and reflex vomiting may be elicited by the stimulation of the gastric afferents carried via vagal and sympathetic nerves to the vomiting center in the brainstem. If gastric stasis and distention are primary causes of nausea and vomiting in diabetic gastroparesis, these symptoms should respond to gastric decompression by either vomiting or by insertion of a nasogastric tube.
Severe nausea and vomiting can limit oral nutrition and be a contributor to morbidity. Gastroparesis also may lead to poor glucose control because of both unpredictable oral intake and poor absorption of nutrients from delayed gastric emptying. When nausea and vomiting are prominent symptoms, some determination should be made regarding whether or not gastroparesis is the primary cause of these symptoms. Although some patients may develop vague upper abdominal discomfort from excessive gastric dilation, when patients complain of marked upper abdominal or midabdominal pain, a search should be made for other GI and abdominal diseases.
Kong et al. (100) studied the natural history of diabetic gastroparesis in a cohort of 86 outpatients with diabetes followed 9 to 14 years later. Of the 86 patients, solid gastric emptying was delayed in 56% and liquid emptying was delayed in 28%. At follow-up, the 21 patients who had died had a greater duration of diabetes and a higher score for autonomic neuropathy than did patients who were alive, but there were no differences in gastric emptying or esophageal transit between the two groups and no evidence that gastroparesis was associated with a poor prognosis overall. Autonomic neuropathy rather than myopathy has long been considered the cause of gastroparesis. However, autonomic dysfunction does not necessarily predict the presence of gastroparesis in patients with type 1 diabetes (101).
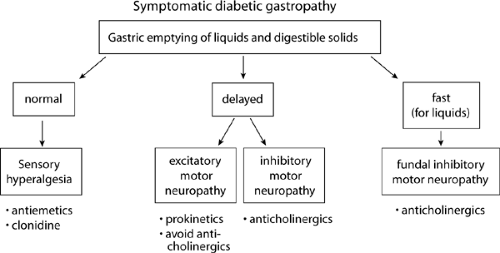
In some patients with diabetes, gastric emptying may fluctuate between normal and delayed. In some patients, nausea and vomiting are associated with episodes of pyloric spasm (87). These abnormalities may cause gastric stasis and the symptoms of nausea and vomiting intermittently. Such patients would be expected to respond to treatments that inhibit abnormal contractions, such as anticholinergic agents, rather than to prokinetic agents (Fig. 64.8). In still other patients with diabetes, nausea and vomiting may be due to an unknown cause unrelated to gastroparesis, and nausea and vomiting may cause, rather than result from, delayed gastric emptying. The vomiting reflex, regardless of activation by central or peripheral mechanisms, produces dysrhythmia of gastric slow waves and inhibition of antral contractions and characteristic retropropulsive motor activities in the small bowel. It is well known that stimulation of the vestibular system by circular vection involving rotation of a drum with alternating dark and light vertical stripes around a subject placed inside the drum leads to development of symptoms of motor sickness. Such a stimulation also causes tachygastria (68). Tachygastria is associated with weakened contractions and delayed gastric emptying (67). If delayed gastric emptying represents a GI motor response to nausea and vomiting, treatment should be directed toward searching for and treating the underlying cause of nausea and vomiting and primary use of antiemetics (Fig. 64.8).
DIABETIC GASTROPATHY WITHOUT DELAYED GASTRIC EMPTYING
Many patients with diabetes manifest symptoms of gastropathy without associated delayed gastric emptying. The symptoms of
this group of patients do not distinguish them from those with delayed gastric emptying. Some patients with diabetes, and particularly obese patients with type 2 diabetes, may complain of early satiety but have accelerated gastric emptying. These patients show no evidence of autonomic neuropathy (102). However, this symptom complex is thought to be due to loss of nitrergic innervation of the gastric fundus in patients with diabetes, which impairs receptive relaxation and accommodation reflexes. Nausea and vomiting are also common complaints in patients with diabetes, occurring in almost one third (72). Nausea and vomiting are common symptoms during acute ketoacidosis, and in most patients they subside with treatment of the acute metabolic abnormality. Sometimes they occur in a chronic pattern of daily symptoms that wax and wane in severity. In a minority of symptomatic patients with diabetes, the pattern is paroxysmal; such patients may have varying periods with minimal or no symptoms, only to be unexpectedly disabled by the sudden onset of severe nausea and vomiting, necessitating hospitalization for dehydration and ketoacidosis.
this group of patients do not distinguish them from those with delayed gastric emptying. Some patients with diabetes, and particularly obese patients with type 2 diabetes, may complain of early satiety but have accelerated gastric emptying. These patients show no evidence of autonomic neuropathy (102). However, this symptom complex is thought to be due to loss of nitrergic innervation of the gastric fundus in patients with diabetes, which impairs receptive relaxation and accommodation reflexes. Nausea and vomiting are also common complaints in patients with diabetes, occurring in almost one third (72). Nausea and vomiting are common symptoms during acute ketoacidosis, and in most patients they subside with treatment of the acute metabolic abnormality. Sometimes they occur in a chronic pattern of daily symptoms that wax and wane in severity. In a minority of symptomatic patients with diabetes, the pattern is paroxysmal; such patients may have varying periods with minimal or no symptoms, only to be unexpectedly disabled by the sudden onset of severe nausea and vomiting, necessitating hospitalization for dehydration and ketoacidosis.
Even without ketoacidosis, patients with diabetes experience nausea and vomiting that correlate poorly with delayed gastric emptying (22). Several physiologic factors may play a role, including decreased compliance of the proximal stomach, which may account for an increased perception of gastric distention in patients with type 1 diabetes (103). Moreover, blood glucose concentrations appear to affect the perception of sensations arising from the GI tract; during hyperglycemia, gastric distention in healthy subjects produced more intense nausea and fullness (104,105). However, it is unclear whether physiologic changes in blood glucose levels directly alter sensation.
DIAGNOSIS OF DIABETIC GASTROPATHY
The presenting symptoms of diabetic gastropathy are nonspecific and also could be caused by mechanical obstruction of the gut, peptic ulcer disease, gastroesophageal reflux, chronic cholecystitis, pancreatitis, or metabolic conditions such as uremia, hypercalcemia, hypokalemia, hypocortisolemia, hypothyroidism, or pregnancy. Abdominal pain is generally not considered a significant feature of the clinical presentation of diabetic gastropathy. The relative lack of symptoms in patients with diabetes has been attributed, in part, to afferent neuropathy (106). However, one study showed that abdominal pain was a feature in almost 90% of patients with diabetic gastroparesis. The pain was described as burning, vague, or crampy. Only 36% localized to the upper abdomen. In all, 60% of patients complained of postprandial pain, whereas 80% complained of nocturnal pain that interfered with sleep. Generally, pain responded poorly or not at all to prokinetic agents (107). Nausea or vomiting in the morning, particularly with cranial neurologic symptoms, is an important indicator for considering central nervous system and metabolic disorders. A number of medications, including antidepressants with anticholinergic properties, can also slow gastric emptying. Eating disorders such as anorexia nervosa may be present, particularly in adolescents. Vomiting of old food suggests either gastroparesis or gastric outlet obstruction. An approach to the evaluation of patients with suspected diabetic gastropathy is detailed in Table 64.2.
TABLE 64.2. Investigations in Suspected Diabetic Gastropathya | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Delayed gastric emptying is the hallmark test of diabetic gastroparesis. A number of different methods are used for measuring gastric emptying in humans. The method of choice depends on whether solid or liquid meals are to be studied, the level of precision required, the degree of invasiveness that the subject or patient will tolerate, ethical considerations, and the local facilities and expertise available. Simple studies such as a plain abdominal film and an upper GI series help identify patients with gastric bezoar and major problems of gastric stasis. Barium studies and esophagogastroduodenoscopy help exclude mechanical causes of gastric outlet obstruction. Gastric emptying of indigestible solids can be determined by studying the emptying of radiopaque markers from the stomach with serial abdominal radiographs (96).
There is no true “gold standard” in this field, but scintigraphy, with appropriate labeling of the test-meal components and appropriate corrections applied to the obtained images, is still the choice and most widely used diagnostic tool in the clinical setting. Emptying of both solid and liquid meals can be assessed simultaneously with the use of dual markers: a 99Tc-labeled solid phase and an 111In-labeled liquid phase (99). Most other techniques are compared with this method, but its application is limited by the need to restrict exposure to ionizing radiation. Clinically, a variety of different meals are used to measure gastric emptying of solids. Because both the physical state of the labeled meal and its nutritional composition determine the rate of emptying in control subjects, the degree of sensitivity of different test meals in detecting abnormalities in diabetes varies.
The most sensitive technique for the measurement of gastric emptying involves the use of chicken liver labeled with 99Tc-sulfur colloid by either in vivo or in vitro methods (61). The labeled liver is then cooked and incorporated into a complex meal. This technique appears to yield the most stable bonding of the radionuclide to solids. In healthy subjects, the labeled meal is emptied in two phases, a lag phase, which appears to correlate with antral grinding of the meal, and a postlag phase of emptying, which represents the passage of the dispersed chyme from the stomach. However, the more involved process of preparing the meal and issues of palatability have perhaps hindered its wide acceptance. More commonly, a less sensitive technique is used that binds the 99Tc-sulfur colloid with cooked eggs, either scrambled or in an egg salad sandwich (70). This test meal usually empties in a single linear phase, because the binding of label to solid is not as stable, and some of the label begins emptying early with the liquid phase.
Gastric emptying is highly variable in patients with diabetes. Some patients with diabetes—particularly those with nausea and vomiting—have evidence of delayed gastric emptying, whereas other patients with diabetes may in fact exhibit accelerated gastric emptying. Presence or absence of symptoms of
upper GI dysfunction correlated poorly with objective measures of gastric emptying in subjects with type 1 diabetes. Nowak et al. (108) studied 21 patients with type 1 diabetes by solid-phase gastric-emptying scintiscan. Thirteen patients had symptoms of GI dysfunction (nausea, vomiting, early satiety, or constipation), and eight patients had no GI symptoms. Eleven patients had orthostatic hypotension. As a group, the patients with diabetes showed gastric emptying that was not significantly different from that of 12 healthy control subjects. Those patients with diabetes without GI symptoms and without orthostatic hypotension, however, showed a gastric emptying half-time that was significantly faster than that of the control subjects. Conversely, those patients with diabetes with nausea, vomiting, and early satiety (or early satiety alone) showed emptying times that were significantly greater than those of the patients with diabetes without these symptoms. No correlation was found between the gastric emptying and the duration of diabetes, the fasting blood glucose at the time of the study, or the respiratory variation in heart rate. These observations indicate that highly variable rates of gastric emptying occur in patients with type 1 diabetes and that accelerated gastric emptying may occur in patients with diabetes who have no symptoms of GI dysfunction.
upper GI dysfunction correlated poorly with objective measures of gastric emptying in subjects with type 1 diabetes. Nowak et al. (108) studied 21 patients with type 1 diabetes by solid-phase gastric-emptying scintiscan. Thirteen patients had symptoms of GI dysfunction (nausea, vomiting, early satiety, or constipation), and eight patients had no GI symptoms. Eleven patients had orthostatic hypotension. As a group, the patients with diabetes showed gastric emptying that was not significantly different from that of 12 healthy control subjects. Those patients with diabetes without GI symptoms and without orthostatic hypotension, however, showed a gastric emptying half-time that was significantly faster than that of the control subjects. Conversely, those patients with diabetes with nausea, vomiting, and early satiety (or early satiety alone) showed emptying times that were significantly greater than those of the patients with diabetes without these symptoms. No correlation was found between the gastric emptying and the duration of diabetes, the fasting blood glucose at the time of the study, or the respiratory variation in heart rate. These observations indicate that highly variable rates of gastric emptying occur in patients with type 1 diabetes and that accelerated gastric emptying may occur in patients with diabetes who have no symptoms of GI dysfunction.
Alternatives to scintigraphy are tracer methods that indirectly measure gastric emptying by assessing the time it takes for ingested marker substance to appear in either the blood or the breath, such as following the appearance in blood of paracetamol (109) or octanoin (110). These methods assume that there is no barrier either to the absorption of the tracer or to its metabolism and require normal intestinal absorption, liver metabolism, and lung function (for breath testing). Such techniques may be useful for screening in large populations, but they require sampling over several hours, are cumbersome, require complicated calculations, and possibly are too inaccurate for clinical use. However, a simplified nonradioactive assessment of solid gastric emptying has recently been proposed that uses a [13C]Spirulina platensis breath test that results in a half-life for solids comparable with those obtained by scintigraphy (110).
The double-sampling gastric aspiration technique is used mainly for physiologic investigations in the research laboratory. It allows serial measurements of the composition of the gastric contents and of the volume and composition of gastric secretions but can be used only with liquid meals. Other imaging tools such as ultrasonography and epigastric impedance measurements produce results that correlate well with those obtained by scintigraphy or aspiration. The main advantage of ultrasonography is that it is noninvasive and does not require radioactive isotopes. It can provide information on gastric emptying rate, on antral diameter, and in duplex mode, on motility and transpyloric flow, particularly of liquids. However, it is time consuming, less accurate in obese patients or for solid emptying, and requires a skilled operator. Moreover, the presence of large amounts of gas in the stomach may hinder the study (111). Epigastric impedance to electrical current increases after drinking liquid with low conductance, such as water. Subsequent decline in impedance reflects the duration of gastric emptying. Although electrical impedance measures are noninvasive, they cannot be used for solid or semisolid meals and are sensitive to body movements. Nevertheless, impedance has been applied to documenting improvement in gastric emptying with metoclopramide and may help in confirming or ruling out the presence of gastroparesis (112). Magnetic resonance imaging (MRI) allows the physician to follow the rate of gastric emptying while simultaneously observing any morphologic abnormalities that may contribute to abnormal gastric function. However, this assessment is time consuming and expensive and remains an investigative research tool (113).
Other noninvasive tools include breath hydrogen testing (114) and the potato-lactulose breath test (115), which have been reported to identify patients with diabetic gastroparesis. A metal-detector test has also identified transit disorders in different GI segments of patients with diabetes mellitus. (116).
Regardless of the method used, the clinician must be aware of the intersubject variability for each test in healthy individuals and of the factors known to influence the gastric pattern.
TREATMENT OF DIABETIC GASTROPATHY
Treatment of diabetic gastropathy entails improving gastric emptying, when abnormal, and improving symptoms. The principles of treatment are (a) acute management; (b) dietary adjustments; (c) use of gastric prokinetic agents that enhance gastric emptying; (d) treatment of associated conditions; and in severe cases, possibly (e) feeding jejunostomy or surgery.
Acute Management
In situations in which severe episodes of protracted vomiting are associated with dehydration and diabetic ketoacidosis, hospitalization is mandatory. In such cases, it is essential that the patient be fasted, and the passage of a nasogastric tube may be necessary. Intravenous fluid should be administered, and insulin should be given as indicated by the serum levels of glucose and ketones.
Dietary Adjustments
Dietary adjustments should be made in all patients with symptomatic gastroparesis. A standard American Diabetes Association diet may be poorly tolerated. The recommended diet should be low in fiber and fat and administered in frequent, small feedings (117). It may be necessary to obtain caloric counts to determine the adequacy of nutritional intake. If caloric intake is inadequate, the diet can be supplemented with high-calorie liquid supplements. Because liquids are emptied more easily from the gastroparetic stomach, these will be better tolerated. Some patients may even find it more practical to ingest the majority of their nutrition as liquid supplements. A three-step dietary program has been advocated (Table 64.3) (70).
TABLE 64.3. Three-step Program in Treatment of Severe Gastroparesis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Gastric Prokinetic Agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree