VACCINES FOR VIRAL DISEASES WITH SIGNIFICANT CENTRAL NERVOUS SYSTEM MANIFESTATIONS
DAVID W. KIMBERLIN
Prevention of disease through deliberate exposure of susceptible human hosts to infectious agents of decreased virulence dates back a millennium. The pace of discovery and technological advances of vaccination development, however, have accelerated dramatically over the past 60 years, with the number of diseases for which vaccines are routinely used in prevention now approaching two dozen. This chapter focuses on vaccines that protect against viral infections that have significant central nervous system (CNS) manifestations, including measles, mumps, poliomyelitis, rabies, and Japanese B encephalitis. Given the scope of this textbook, discussion of each of these diseases is limited to neurologic manifestations of each wild type disease, followed by a comprehensive review of vaccine prevention.
MEASLES
Neurologic Manifestations of Measles
Central Nervous System Involvement During Acute Disease
First described at the end of the eighteenth century (1), measles encephalitis causes the greatest morbidity and mortality of all the complications of measles infection. Symptoms of encephalitis begin within 8 days of the onset of illness, most often during the exanthem period (2,3). Presenting symptoms include seizures (56%), lethargy (46%), coma (28%), and irritability (26%) (3). Analysis of cerebrospinal fluid (CSF) usually yields a mild pleocytosis with a slightly elevated protein level and normal glucose concentration (3,4). The CSF can be completely lacking in signs of inflammation in cases of measles encephalitis (3).
A Centers for Disease Control and Prevention (CDC) study of reported cases of measles encephalopathy in the United States between 1962 and 1979 revealed a 15% mortality rate associated with this complication (5). The investigators found that 25% of survivors of measles encephalopathy had severe sequelae, including mental retardation, seizures, severe behavioral disorders, deafness, hemiplegia, and paraplegia. There were 0.73 cases of encephalitis per 1,000 estimated total cases of measles, and the death rate due to measles encephalitis was 1 per 10,000 reported cases of measles. Other investigators have reported neurologic sequelae in as many as 50% to 60% of cases of measles encephalopathy (2).
The mechanism by which measles virus causes encephalitis is incompletely understood. Neurologic involvement may entail direct viral-induced cellular damage; alternatively, an autoimmune-mediated process of tissue damage is possible. Some investigators have successfully identified viral RNA or antigens in the CNS of affected patients (4,6), whereas others have been unable to demonstrate a direct viral involvement (7–10). Both mechanisms may contribute to the pathology present in measles encephalitis.
Immunocompromised patients may develop an unusual form of acute progressive encephalitis, formerly known as measles inclusion body encephalitis (MIBE). Following an incubation period lasting from 5 weeks to 6 months, illness often initially manifests with seizure activity. Hemiplegia, slurred speech, stupor, coma, and hypertonia can develop. This disorder is usually fatal, with death occurring within 1 week to 2 months following the onset of neurologic findings (11,12). It is often associated with malignancies of the lymphatic or reticuloendothelial systems (e.g., leukemia or lymphoma) (13). Electron-microscopic studies of brain specimens from these patients demonstrate nucleocapsid structures in the cytoplasm of infected cells; however, the viral budding that can be seen in acutely infected cells is not present (13). Infectious virus has been isolated infrequently from these patients (14).
Subacute Sclerosing Panencephalitis
Subacute sclerosing panencephalitis (SSPE) is a rare degenerative neurologic disease that occurs years after measles infection. The disease was first described clinically in the 1930s (15). Since its initial description, it has undergone many name changes, including “lethargic encephalitis with inclusions,” “subacute inclusion body encephalitis” (15), and “subacute sclerosing leukoencephalitis” (16,17). The term SSPE was coined in 1950 by Greenfield (16) to stress that both the white and the gray areas of the brain are affected.
SSPE clinically is very similar to MIBE. However, the asymptomatic period following the acute measles infection is much longer in SSPE. Additionally, whereas SSPE patients often mount a strong immune response to most measles virus proteins (with the exception of the M protein), MIBE patients have a diminished antibody response to measles virus antigens (13,18,19).
Patients afflicted with SSPE are usually children or young adults. Reported patient ages range from 1 to 35 years old, with an average age at onset of about 9 years (20–22). There is a 2.3:1 male-to-female prevalence ratio (20). More than half of all cases occur in patients who had their typical measles infection when they were younger than 2 years of age (20–22). Prior to widespread measles immunization in the United States, there was a higher prevalence in whites and among rural populations, and a higher prevalence in the southeastern portion of the United States (20). Worldwide annual incidence figures have been estimated at 1 case per million persons per year (21,22); in the United States, there are 0.35 cases per million persons per year (20). For every 100,000 cases of measles that occur naturally worldwide, there are 0.6 to 2.2 cases of SSPE (20,23–25).
At initial presentation, patients with SSPE demonstrate subtle changes in mental status, followed by delirium, dementia, myoclonus, motor incoordination, seizures, and visual and speech impairment; disease then progresses to stupor, mutism, coma, and death (21,22,26–28). Clinical staging of SSPE was established by Jabbour (29), as follows:
1. Stage 1 manifests as cerebral changes and lasts 1 to 2 months.
2. Stage 2 involves worsening convulsions and lasts 2 to 3 months.
3. Patients in the third stage (coma and opisthotonus) exhibit worsening neurologic status over 1 to 4 months.
4. The final stage of SSPE involves autonomic dysfunction and lasts from months to years.
Pathologic evaluation of the brains of patients with SSPE invariably reveals gray matter involvement, occasionally with extensive white matter sclerosis. Intranuclear and intracytoplasmic inclusion bodies are seen in the affected neurons. These “Cowdry inclusion bodies” have been shown to contain measles virus (30).
The diagnosis of SSPE often can be made on the basis of information obtained on the clinical and laboratory evaluation. The typical electroencephalographic (EEG) pattern of SSPE consists of periodic bilateral bursts of high-voltage activity every 3 to 20 seconds with background suppression (27). Computed tomography (CT) scan reveals atrophy involving the cerebrum, cerebellum, and brainstem; in addition, diffuse white matter involvement can be seen, as can involvement of the thalamic and lentiform nuclei. The correlation between the clinical staging and magnetic resonance imaging (MRI) usually is poor (31), although diffusion-weighted MRI has been used to differentiate between stages of SSPE (32,33). The CSF protein is elevated because of the presence of oligoclonal immunoglobulin G (IgG) directed against measles antigens (13,18).
Although the specific pathogenic mechanisms in SSPE have yet to be elucidated, numerous reports have detected measles virus in brain biopsy specimens from patients with SSPE (13,18,30,34–37). Intranuclear and cytoplasmic inclusion bodies can be detected in neurons, astrocytes, and oligodendrocytes; additionally, nucleocapsid particles can be visualized in these inclusion bodies by electron microscopy (17,38–40). In contrast to electron microscopy of infected cells from patients with acute measles infection, nucleocapsids are not visualized assembling or budding out from the cell surface in specimens from patients with SSPE. Investigators have speculated that a block in the formation of competent viral particles may exist in patients with SSPE (41). In support of this hypothesis, cell-free extracts of brain tissue from patients with SSPE are not infectious for susceptible cells in tissue culture as assayed by cytopathic effect, hemagglutination, and fluorescent staining. When brain tissue is co-cultivated with tissue culture cells (e.g., HeLa cells and Hep II cells), infection of the tissue culture cells occurs, indicating that the block is reversed by the presence of nonneural cell types (30).
Analysis of brain tissue specimens from patients with SSPE using monoclonal antibodies directed against the six measles virus proteins revealed that whereas all the proteins are found in the various brain tissue samples, all six proteins were never found simultaneously in a single tissue specimen. In other words, one protein would be missing in one sample and another would be missing in a different sample (13,18,34,37,42,43). In contrast, all six proteins can be demonstrated simultaneously in cells infected with measles virus in tissue culture. Investigators have occasionally described more than one profile of proteins present in different areas of the same brain specimen (43). Of the six viral proteins, M protein is present least often. Of note, M protein is involved in viral assembly and budding. Analysis of messenger RNA (mRNA) from SSPE specimens has revealed a high mutation rate in all of the viral proteins (43–46). Although these mutations are not seen in viruses isolated during acute infections, they are thought to occur with the same regularity as in SSPE but are simply selected against in the acute infection. Selection pressures in nondividing neural tissues may be less than those in actively dividing tissues, and such differences may permit viruses with defects to survive (46). In fact, viruses with “defects” that prevent them from acutely lysing neuronal cells may actually have a selective advantage, in that they remain “hidden” from the immune system when they reside solely within the cells (43).
Reports have been published suggesting that inosiplex (isoprinosine) (47), inosiplex plus interferon-β (48), inosiplex plus interferon-α plus lamivudine (49), intraventricular ribavirin (50), interferon-β monotherapy (51), and interferon-α monotherapy (52) may be effective in slowing the progression of SSPE. However, these studies have not been controlled. One international multicenter controlled study compared oral inosiplex with oral inosiplex plus intraventricular interferon-α, and found that neither group was superior but that higher proportions of both treatment groups stabilized or improved (34% and 35%, respectively) compared to the spontaneous remission rates of 5% to 10% reported in the literature, suggesting that treatment was superior to no treatment (53).
Measles vaccination programs provide the best means by which the incidence of SSPE can be reduced. The risk of SSPE in vaccinated children (0.5 to 1.1 cases per million per year) is less than that in children who have had natural measles infection (5.2 to 9.7 cases per million per year) (20). In addition, the incidence of SSPE has fallen dramatically since the initiation of vaccination programs, suggesting that the vaccine is protective.
VACCINATION AGAINST MEASLES
History
The progenitor measles strain for most of the current vaccines in use worldwide was isolated from a patient named David Edmonston. Within 9 years of this initial viral isolation, two measles vaccines were licensed for use in the United States in 1963. From 1963 to 1968, an estimated 1.8 million doses of a formalin-inactivated, alum-precipitated measles vaccine were administered to 600,000 to 900,000 persons in the United States (54). The short-lived immunity conferred by this vaccine and the propensity of patients to develop atypical measles infections led to the cessation of its use after 5 years (55). The second measles vaccine licensed in 1963 was the attenuated, live-virus Edmonston B vaccine. In use from 1963 to 1975, 18.9 million doses of this vaccine were administered in the United States (26,28). Although the protection conferred by this live-virus vaccine was superior to that of the killed vaccine, side effects such as fever and rash were very common. Although simultaneous administration of small doses of immune globulin decreased the occurrence of such reactions, the Edmonston B vaccine was eventually replaced by additionally attenuated live-virus vaccines with fewer side effects.
Several further attenuated strains of measles virus have been derived from the original Edmonston strain. These vaccines confer good protection against natural disease while causing fewer side effects, thereby eliminating the need for the simultaneous administration of immune globulin. The Schwarz strain was administered in the United States from 1965 through 1976, and it is still used in most parts of the world. The Moraten (more attenuated Enders) strain has been licensed for use in the United States since 1968, with more than 165 million doses having been administered since that time (56,57). The Moraten vaccine is the only measles vaccine licensed for use in the United States. Combination vaccines that include mumps, rubella, and measles (the measles-mumps-rubella [MMR] vaccine) and mumps, rubella, measles, and varicella (MMRV vaccine) have been available since 1971 and 2005, respectively. Monovalent measles vaccine has been withdrawn from the U.S. market, leaving MMR and MMRV as the only measles-containing vaccines available in this country. Vaccines using other strains of measles virus are widely used throughout the world and are usually comparable to the Moraten vaccine available in the United States (58).
Measles Vaccination Today
The dramatic decrease in cases of measles since the licensure of the measles vaccine is a testament to its success. The incidence of measles has decreased more than 99% since the introduction of measles vaccine in the United States in 1963 (59). The last major resurgence of measles in the United States occurred from 1989 to 1991, during which time the incidence of measles increased sixfold to ninefold over the median annual incidence earlier in the 1980s, with 120 reported measles-related deaths (57,60). At the peak of the epidemic in 1990, the incidence of measles among children younger than 5 years of age was 15-fold higher than the median annual incidence reported from 1981 through 1988 (57). This measles resurgence was primarily a consequence of the failure to vaccinate preschool-aged children according to the recommended immunization schedule (61). With the implementation of the two-dose measles vaccination schedule discussed later in this chapter, the incidence of measles in the United States has decreased to a record low, with only 86 cases being reported in 2000 (62) and a reported incidence of less than 1 case per million since 1998 (63), culminating in the elimination of endemic transmission being declared in 2000 (64). After the elimination of measles was achieved, a number of outbreaks of limited size occurred as a result of importation into primarily unvaccinated populations; these outbreaks have not extended to the highly two-dose vaccinated populations (65). Each year since 2000, an average of 60 people in the United States are reported to have measles. In 2011, the number of reported cases was higher than usual, with 222 people developing the disease. Nearly 40% of these people acquired measles in other countries, including countries in Europe and Asia, and then brought it to the United States and spread it to others, resulting in 17 measles outbreaks in various U.S. communities (66).
A notable recent development in the worldwide effort to prevent measles infection involved high-titer live attenuated measles vaccine. Several studies in the late 1980s found that high-titer (>104.7 log10 infectious units) Edmonston-Zagreb live attenuated measles virus vaccine induced serologic response rates among young infants that were comparable to the response rates following standard titer vaccines administered at 9 months of age (67–71). However, higher than expected mortality rates later in infancy among recipients of these high-titer vaccines have been reported in areas where mortality rates from measles are high among children younger than 9 months (72–74). Of note, these deaths were due to common childhood illnesses, not measles infection (73,74). Investigators initially hypothesized that in a manner similar to natural measles infection, immune suppression induced by the high-titer vaccine predisposes these infants to severe life-threatening infections with common childhood pathogens (75–78), but more recent analyses suggest that the sequence of other vaccines administered concomitantly with high-titer Edmonston-Zagreb measles vaccine, rather than the high-titer vaccine itself, accounts for the increased female mortality in these trials (79). These high-titer vaccines were never licensed in the United States and are no longer in use in foreign countries (58). Importantly, increased mortality rates have not been noted among recipients of standard-dose measles vaccine, including standard dose Edmonston-Zagreb vaccine. Studies of standard dose Edmonston-Zagreb vaccine given at 4.5 months and 9 months of age suggest that early measles immunization has beneficial nonspecific effects on children’s survival, particularly for girls and for children who have not received neonatal vitamin A (80).
Vaccine Recommendations
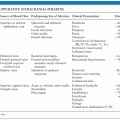
Prior to 1989, the measles elimination strategy called for administration of one dose of measles vaccine at 15 months of age. Because of the increase in measles cases among adolescents and young adults, both the CDC Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics (AAP) Committee on Infectious Diseases recommended in 1989 that a routine two-dose measles vaccination schedule be adopted (58,81). The rationale behind such a recommendation is that the two-dose schedule may successfully protect those patients who failed to respond adequately to a single dose of measles vaccine (primary vaccine failures); in addition, patients who missed their single-dose immunization are more likely to be detected and vaccinated under a two-dose vaccination program. Table 50.1 lists the current recommendations for measles vaccination.
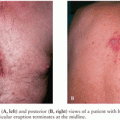
Adverse Reactions to Measles Vaccine
Between 5% and 15% of measles vaccine recipients develop fever with a temperature of 39.4°C or higher between 5 and 11 days postvaccination, presumably as a reaction to replication of the live attenuated virus and usually without additional symptoms. About 5% of all recipients of measles vaccine develop a transient rash. True encephalitis or encephalopathy occurs in vaccine recipients at a rate equal to or lower than that seen in unvaccinated or baseline populations (<1 case per million doses of vaccine), and such neurologic sequelae have not been causally associated with vaccine administration (56). Postvaccination seizures have been reported in children, coinciding with the occurrence of fever (“febrile seizures”). Among 12- to 23-month-old recipients of MMR and varicella vaccines administered concurrently but at separate sites, 3 to 4 febrile seizures occur per 10,000 children vaccinated; for children of the same age range receiving MMRV, 7 to 9 febrile seizures occur per 10,000 children receiving MMRV (82). Thus, one additional febrile seizure is expected to occur per approximately 2,300 to 2,600 children 12 through 23 months old vaccinated with MMRV, when compared with separate MMR and varicella vaccine administration. The period of risk for febrile seizures is from 5 through 12 days following receipt of the vaccine(s). No increased risk of febrile seizures is seen among patients 4 to 6 years of age receiving MMR, MMRV, or monovalent varicella vaccines. Febrile seizures do not predispose to epilepsy or neurodevelopmental delays later in life and are not associated with long-term health impairment. The AAP recommends that either MMR and varicella vaccines separately or MMRV vaccine be used for the first dose of measles, mumps, rubella, and varicella vaccines administered at 12 through 47 months of age (82). Transient thrombocytopenia has been reported following administration of the MMR vaccine (83).
Allergic reactions to the vaccine occur only very rarely. The vaccine virus is grown in avian embryos, so trace amounts of egg proteins may be present in the final product. Patients who have demonstrated prior severe egg allergies or who have a history suspicious for egg allergy may be screened by skin testing with the vaccine prior to its use (84–87). Of the hundreds of millions of doses given in the United States, only five incidences of anaphylactoid reactions with associated respiratory problems have been reported (88).
As with naturally occurring infection, measles vaccination is associated with a transient impairment of cell-mediated immunity, as demonstrated by a blunted cutaneous delayed hypersensitivity reaction to administered antigens (e.g., purified protein derivative of tuberculin). Although not thought to be clinically significant, it should be considered before skin testing these patients, should skin testing be indicated. This response can last as long as 4 to 6 weeks postvaccination (58).
Isolation of vaccine virus from human blood postvaccination has not been reported. Thus, viremia either does not occur in this setting or is present only transiently and at very low levels. No cases of person-to-person transmission of the vaccine strains have been reported, and there is no evidence of shedding of vaccine virus (26,28).
In the late 1990s, allegations arose suggesting that the MMR vaccine causes autism (89,90). The hypothesis of such a causal effect was based on an uncontrolled case report study (89). Subsequently, extensive data evaluating this hypothesis have consistently failed to prove such an association (91–96), leading the Institute of Medicine (IOM) Immunization Safety Review Committee (ISRC) to conclude in a detailed report that multiple studies indicate that there is no scientific basis to support this hypothesis (97). The original paper asserting such an association was retracted when financial conflicts of interest by the primary author were discovered and his license to practice medicine in the United Kingdom was revoked. Similarly, evidence does not support the suggestion that MMR vaccine or wild type measles infection is associated with inflammatory bowel disease or Crohn disease (92,96,98–101).
Vaccine Contraindications
Although no cases of in utero infection due to a vaccine strain of measles virus have been reported, pregnant women or those who may become pregnant within 3 months should not receive measles vaccine, thus avoiding the theoretical risk of fetal infection with the vaccine virus (56).
Endogenous interferon induced during a severe febrile illness has the potential to interfere with the immune responses to vaccination. As such, vaccination of patients with severe febrile illnesses should be deferred pending resolution of the intercurrent illness. However, mild febrile illnesses should not delay immunization. A personal or family (i.e., sibling or parent) history of seizure is a precaution, but not a contraindication, for use of MMRV vaccine due to the slight increase in risk of febrile seizures described previously.
In general, patients with severe immunodeficiencies should not be immunized with live-virus vaccines. Exceptions to this recommendation include asymptomatic human immunodeficiency virus (HIV)–infected children and those with symptomatic infection who are not severely immunocompromised, in whom measles immunization (given as MMR vaccine) is recommended because the risk of severe sequelae (including death) from measles infection is high (58,88,102). Severely immunocompromised HIV-infected infants, children, adolescents, and young adults, as defined by low CD4+ T-lymphocyte counts or percentage of total lymphocytes, should not receive measles virus–containing vaccine because vaccine-related pneumonitis has been reported (103,104).
Worldwide Measles Elimination
Since the institution of vaccination programs in Canada, China, the United Kingdom, and the United States, the incidence of measles has decreased markedly (105–107). In addition, the incidence of SSPE has decreased in the United Kingdom since vaccination regimens were implemented (107), and the incidence of measles encephalitis in the United States has declined (105). Because humans are the only known reservoir for measles virus, worldwide eradication is possible.
To eliminate measles, a sufficient number of the world’s population must be seroprotected. When enough people are immune to infection, the virus will no longer be able to infect the number of susceptible hosts required for its continued spread. Mathematical models based on viral infectivity suggest that no less than 94% of the world’s population must be immune to measles infection to eliminate the virus. Because the available vaccines are not completely effective in eliciting a protective response, approximately 97% to 98% of the world’s population must be vaccinated to produce a population that is 94% protected (26,28). In 1980, before the use of measles vaccine was widespread, an estimated 2.6 million deaths due to measles occurred worldwide (108). With worldwide focus, global mortality attributed to measles has decreased from 733,000 in 2000 to 164,000 in 2008 (109). With the financial and technical support of the Measles Initiative international partnership, all countries except India have achieved the 2010 global goal of reducing measles mortality by 90% (109). Measles elimination has been sustained in the World Health Organization (WHO) Region of the Americas since 2002. Although global challenges remain, including a resurgence of measles in sub-Saharan Africa and to a lesser degree in Europe, these encouraging developments led the WHO in 2008 to evaluate the feasibility of the global eradication of measles. A Global Consultation on the Feasibility of Measles Eradication convened in 2010 and concluded that measles can and should be eradicated and that global eradication by 2020 is feasible (110). A thorough assessment of the current challenges and opportunities was published as a supplement to the Journal of Infectious Diseases in July 2011 (Volume 204, Supplement 1).
MUMPS
Neurologic Manifestations of Mumps
Meningeal viral involvement occurs in about half of all cases of mumps infection, with or without clinical signs of meningismus (111–114). Reports concerning the frequency of CNS involvement in mumps infection date back to the beginning of the twentieth century. In 1902, Monod performed lumbar punctures on eight children with mumps; despite the lack of symptoms suggesting CNS involvement, six of the eight patients had elevated CSF white blood cell (WBC) counts (112,115). Additional studies confirmed the association between mumps and abnormal CSF indices. In the winter of 1937, CSF was obtained from 40 children admitted to Willard Parker Hospital in New York with the diagnosis of mumps (114). Sixteen (40%) of the forty specimens had elevations in CSF leukocyte counts and protein concentrations. Six of these sixteen children had no CNS symptoms, whereas ten had symptoms ranging from mild to severe, including drowsiness, nuchal rigidity, seizures, severe headache, and coma. All recovered without sequelae. In a larger study performed in 1943, 235 (63%) of 372 patients with mumps parotitis had CSF leukocytosis (111). And during an outbreak on an army base in 1948, 26 (34%) of 77 adults who contracted mumps had elevated CSF WBC counts, although only 9 patients had CNS symptoms (111).
Although laboratory evidence of CNS inflammation is present in a large proportion of patients with mumps, clinically apparent meningoencephalitis occurs only rarely, on the order of 2 to 8 cases per 1,000 cases of mumps (116,117). Although CNS manifestations can begin more than a week after the onset of parotitis, initial symptoms precede or coincide with parotid swelling in about two thirds of all cases (113,114,118–121). Virus can be isolated from the CSF in the latter instances (122) and histology shows neuronal breakdown (119,123), suggesting that active viral replication results in the symptomatology and pathologic injury noted in early-onset cases. In late-onset “secondary” or “postinfectious” cases of CNS involvement (112,124,125), neuronal injury and CNS inflammation may be caused indirectly, perhaps through autoimmune mechanisms. In late-onset cases, perivascular leukocytic infiltration and demyelination are present on histologic examination of the brain (119,123,124).
Patients with clinically apparent meningitis or meningoencephalitis typically have a moderate elevation of the CSF WBC count (from 500 to 1,000 cells/mm3) with a lymphocyte predominance. The CSF protein level is usually normal or mildly elevated, and CSF glucose concentration is normal.
Although studies vary with regard to estimates of sequelae following mumps meningitis or meningoencephalitis, adverse neurologic outcomes probably occur in fewer than 1% of cases with CNS symptoms (125–128). Ataxia, flaccid paralysis, incontinence, and behavioral changes have all been described in patients with residual neurologic findings (120–122,129). Fatal cases have been reported after mumps meningoencephalitis (119,124).
VACCINATION AGAINST MUMPS
History
A formalin-inactivated mumps vaccine was first tested in 1950, but it had a low protective efficacy against clinical mumps among susceptible persons (130–132). In addition, protection conveyed by this killed vaccine persisted for less than 1 year, necessitating reimmunization. As a result of these shortcomings, the vaccine was discontinued in 1976 (130,132). The attenuated mumps strain used in the vaccine currently licensed in the United States was derived from a child named Jeryl Lynn; this strain was attenuated by serial passage through embryonated eggs by Maurice Hilleman, Jeryl Lynn’s father. Over his career, he developed or substantially improved more than 25 vaccines, including 9 of the 14 now routinely recommended for children, saving literally millions of lives (133). In the first 20 years following the 1967 licensure of mumps vaccine in the United States, an excess of 80 million doses of the Jeryl Lynn strain of attenuated mumps virus vaccine were administered (130,132,134). After implementation of the one-dose mumps vaccine recommendation in 1977, the incidence of mumps in the United States declined from an incidence of 50 to 251 per 100,000 in the prevaccine era to 2 per 100,000 in 1988. After implementation of the two-dose MMR vaccine recommendation in 1989 for measles control, mumps further declined to extremely low levels, with an incidence of 0.1 per 100,000 by 1999. From 2000 to 2005, there were fewer than 300 reported cases per year (incidence of 0.1 per 100,000), representing a greater than 99% reduction in disease incidence since the prevaccine era. Since 2006, though, a series of large-scale mumps outbreaks have occurred. These outbreaks have occurred in communities with high intensities of person-to-person exposure, such as colleges and religious groups. The effectiveness of MMR vaccine to prevent mumps has been estimated at medians of 78% (range: 49% to 91%) for one dose and 88% (range: 66% to 95%) for two doses (135). In response to these outbreaks, third doses of MMR vaccine have been administered. A small study demonstrated an anamnestic response following a third MMR dose within 7 to 10 days after vaccination (136). Two efficacy studies of use of a third dose of MMR to stem a mumps outbreak have been published (137,138). However, in both, the intervention with a third dose of vaccine occurred after the peak of the outbreak. Both also had small numbers of cases postintervention, and for these reasons, the results have limited generalizability. Administration of a third MMR dose in an outbreak setting appears to be safe (139). As of early 2013, available data are insufficient to recommend for or against the use of a third dose of MMR vaccine for mumps outbreak control. Because control measures for mumps are limited, the ability to offer a third dose of MMR vaccine might be a tool that could be used in an attempt to limit the extent of mumps outbreaks, particularly in high-risk settings.
Following vaccination with the Jeryl Lynn strain, the frequency of such postvaccination CNS complications as neuritis, encephalitis, and deafness is no higher than in unvaccinated populations (130,132,140). However, aseptic meningitis as a complication of vaccination with the Urabe Am9 strain of attenuated mumps virus has been widely reported (141–144). This complication occurs 14 to 28 days postvaccination, with an estimated incidence of 1 case in 11,000 to 14,000 doses (143,144). Importantly, the Jeryl Lynn strain mumps vaccine, which is the mumps component of the MMR vaccine, is not associated with cases of aseptic meningitis (145). The meningitis associated with the Urabe Am9 strain is usually mild, resolving without sequelae (141). Although the Urabe strain has a higher efficacy than the Jeryl Lynn vaccine (146), this increased risk of vaccine-associated complications has resulted in its withdrawal from the market in many countries (142).
No cases of person-to-person spread of the Jeryl Lynn mumps vaccine strain have been reported (147). However, at least one case of transmission of the Urabe mumps virus following immunization has been documented (148).
Vaccine Recommendations
Two doses of mumps vaccine are recommended for children in the United States. MMR and MMRV are both options for mumps vaccine administration, but monovalent mumps vaccine no longer is available in the United States. The first dose of MMR or MMRV vaccine should be given routinely to children at 12 through 15 months of age, with a second dose of MMR or MMRV vaccine administered at 4 through 6 years of age. The second dose of MMR or MMRV vaccine may be administered before 4 years of age, provided at least 28 days have elapsed since the first dose and the interval between varicella vaccine doses is at least 90 days.
Acceptable evidence of mumps immunity include the following: (a) documentation of age-appropriate vaccination with a live mumps virus–containing vaccine, which is one dose for preschool-aged children, two doses for school-aged children (grades K to 12), and one dose for adults not at high risk; (b) laboratory evidence of immunity; (c) laboratory confirmation of disease; or (d) born before 1957 (149).
Because it is a live attenuated vaccine, mumps vaccination should be deferred in pregnant women or women who plan to conceive within 28 days. MMR and MMRV vaccines are produced in chicken embryo cell culture and do not contain significant amounts of egg white (ovalbumin) cross-reacting proteins. Children with egg allergy are at low risk of anaphylactic reactions to MMR or MMRV vaccine. Skin testing of children for egg allergy is not predictive of reactions to MMR or MMRV vaccine and is not required before administering MMR vaccine. Live mumps vaccine should be given at least 2 weeks before or at least 3 to 11 months after administration of immune globulin, depending on the dose of immune globulin administered. Patients with immunodeficiency diseases and those receiving immunosuppressive therapy or who are otherwise immunocompromised should not receive mumps vaccine. An exception to this is the patient with HIV infection who is not severely immunocompromised (i.e., for persons aged 5 years or younger: must have CD4 percentages ≥15% for ≥6 months; for persons aged older than 5 years: must have CD4 percentages ≥15% and CD4 ≥200 lymphocytes/mm3 for ≥6 months), in whom MMR vaccine should be given for the measles protection it conveys. Children with minor illnesses with or without fever, such as upper respiratory tract infections, may be immunized. Fever is not a contraindication to immunization. However, if other manifestations suggest a more serious illness, the child should not be immunized until recovered.
Mumps vaccination is followed by a lag of up to a few weeks before antibodies are detectable. As a result, vaccination will not protect susceptible persons recently exposed to mumps virus (131). No data exist to support the use of immune globulin in exposed susceptible persons in either preventing or ameliorating disease (130,132,150,151).
JAPANESE ENCEPHALITIS VIRUS
Neurologic Manifestations of Japanese Encephalitis Virus
Although only a small proportion of patients infected with Japanese encephalitis (JE) virus will be symptomatic, these patients very often will be severely affected and can suffer permanent CNS damage or death. Inoculation occurs with the bite of an infected mosquito. During the following 4- to 14-day incubation period, most persons clear the infection and have no further signs of illness. Those patients who fail to eliminate the virus, however, enter the prodromal stage of illness. This period lasts 2 to 3 days, during which time patients suffer headache, anorexia, nausea, vomiting, and abdominal pain. Low-grade fever and CNS changes ranging from mild disorientation to frank psychosis can also occur during the prodromal stage (152,153).
The acute stage of symptomatic JE virus infection lasts 3 to 7 days. High fever often signals the onset of the acute stage. Seizures occur in about 20% of children during the acute stage, but convulsions develop only rarely in adults. Rapid fluctuations in CNS signs can manifest as hyperreflexia followed quickly by hyporeflexia. Alterations in mental status may produce confusion, disorientation, delirium, or coma. Meningismus, diarrhea, oliguria, and bradycardia can occur during this period. CSF examination reveals an elevated protein concentration and a moderate pleocytosis with between 100 and 1,000 cells/mm3; the polymorphonuclear predominance that occurs early is followed by a shift to a lymphocyte predominance. Fatal cases progress to coma and death within 10 days of the onset of the acute stage (152,153). Mortality rates range from 20% to 30%.
Patients who survive the acute stage subsequently enter the subacute stage followed by the convalescent stage. The former lasts from the second to the fourth week and is marked by gradual improvement in mental status. During this subacute period, complications such as pneumonia, pressure ulcers, and urinary tract infections are common. Motor deficits such as spastic paralysis, fasciculation, and extrapyramidal tract abnormalities may develop. The final stage of symptomatic JE virus infection, the convalescent stage, lasts from the fourth to the seventh week. Resolution of the neurologic deficits occurs slowly, if at all. From 30% to 50% of survivors are left with permanent sequelae, including mental retardation, emotional instability, personality disturbances, and motor and speech abnormalities (152–156). Children and elderly adults are more likely to develop encephalitis during JE virus infection than young adults and middle-aged persons (157). The neurologic sequelae following JE virus are age dependent; permanent impairment is much more common in patients younger than 10 years at the time of onset of disease, and infants experience more severe sequelae than older children (152,153).
Infection with JE virus in early pregnancy increases the risk of stillbirth in swine (158). In humans, JE virus has been isolated from brain, liver, and placenta of the products of spontaneous abortion following acquisition of JE virus infection in early pregnancy (158). In addition, cases in which neurologic symptoms occur in newborns following first-trimester maternal JE virus infection have been reported (158).
How much of the pathology that occurs in cases of JE virus is due to direct viral replication in the CNS and how much damage is caused by the host immune response to the virus is not clear. Intracerebral inoculation of JE virus into spider monkeys produces no overt symptoms unless they are given immunosuppressive agents before infection (159). In immunocompetent monkeys, mild gray matter destruction is seen on histologic examination of the brain and spinal cord. Immunosuppressed monkeys, on the other hand, develop severe to overwhelming lesions that produce much neural damage that manifests as severe flaccid paralysis. These findings suggest direct damage of the neurons due to viral replication rather than an immune-mediated process. Clinical correlates also are more consistent with direct viral damage, because most deaths occur during the acute phase when virus can still be cultured from the CSF and brain tissue (155). Histologic examination of human brain tissue from fatal cases reveals severe destruction of the gray matter throughout the brain; tissue damage is especially pronounced in the regions of the basal ganglia, the floor of the fourth ventricle, the cerebellum, and the cerebral cortex. In addition, the spinal cords of such persons are usually diffusely involved (160).
Infection with JE virus is thought to convey lifelong immunity, although waning protection may leave the elderly at risk for reinfection (153,157,161). Prior infection with dengue confers a degree of protection against JE virus, presumably through cross-reacting antibodies (162,163). Minor antigenic drift among wild type JE virus strains has been noted across both time and geographic regions (164,165).
The diagnosis of JE virus is usually made on the basis of serology (152,153). Although laboratory isolation of JE virus from brain specimens in fatal cases is possible, the virus has only rarely been isolated from blood and CSF (166). It is difficult to detect viral antigens in specimens of CSF or serum, although they can be identified by complement fixation following viral growth in tissue culture (152,153). The differential diagnosis for JE virus includes leptospirosis, enteroviral disease, mumps meningoencephalitis, herpes encephalitis, rabies, dengue, bacterial meningitis, cysticercosis, Reye syndrome, neoplasia, toxins, and the postinfectious encephalitides (152,153).
Because of the high prevalence of asymptomatic JE virus infection in some areas, certain populations have high proportions of individuals with JE virus IgG seropositivity. As a result, the diagnosis of acute JE virus infection in patients from endemic regions frequently requires the demonstration of anti–JE virus immunoglobulin M (IgM) antibodies. Simultaneous CSF and serum IgM titers are useful in diagnosing JE virus; patients with acute JE virus have high anti-JE virus IgM titers in the CSF, with CSF titers usually exceeding those in the serum (152,153,156,167). In contrast, CSF anti-JE virus IgM is undetectable or low and serum anti-JE virus IgM is high in cases of acute asymptomatic JE virus infection (167). The CSF IgM titers are prognostic as a high CSF IgM titer equates with a higher survival rate than those who do not mount a strong CSF immune response (168).
In cases of JE virus, therapy consists solely of supportive measures. Although human immune globulin prevents death in experimentally infected animals (169), it has not proven beneficial in the management of human disease. Such passive immunization would probably have to be administered early in infection (before the onset of neurologic symptoms) to be beneficial (152,153).
VACCINATION AGAINST JAPANESE ENCEPHALITIS VIRUS
Three types of killed JE virus vaccines are commercially available. An inactivated vaccine prepared from JE virus grown in mouse brains is manufactured in Japan and Korea; a killed vaccine from virus grown in hamster kidney cells in tissue culture is prepared in China (170); and an inactivated Vero cell culture–derived JE vaccine is manufactured by the Austrian company Intercell. The administration of two doses of the vaccine prepared from mouse brain given 1 month apart results in seroconversion rates of 90% to 100%. The protective efficacy of this vaccine is 80% (170). In comparison, the vaccine prepared in hamster kidney cells has an estimated protective efficacy of 95% (170). For the Vero cell culture–derived vaccine, protective concentrations of neutralizing antibodies were achieved in 58% to 83% of recipients 12 months after a first dose of vaccine and in 100% of recipients 28 days after receipt of a booster dose given at 15 months after the first dose of the primary immunization; neutralizing antibody titers persist for at least 1 year. Side effects of all three vaccines occur in fewer than 1% of recipients and include fever, headache, local pain, swelling, and fatigue; allergic reactions are noted in fewer than 0.02% of vaccinees (152,153).
In China, live attenuated JE virus vaccines have been developed and tested in humans (152,153) and have demonstrated efficacy (171,172) but are not available outside of China. New-generation JE virus vaccines that use attenuated vaccinia vectors expressing viral proteins of JE virus have shown promise in animals (173–175).
The incidence of JE virus infection has decreased markedly in Japan since 1966. This decline is largely due to the widespread vaccination of children. In addition, insect-control measures have decreased the mosquito population, thus limiting the vector required for transmission of JE virus to humans (154). Efforts to limit the reservoir for JE virus have focused on vaccination of domesticated animals. Live attenuated vaccines have been used in swine because killed vaccines do not confer immunity in this species (152,153).
The first JE virus vaccine licensed for use in the United States was a JE virus inactivated vaccine (JE-VAX) derived from infected mouse brains by the Japanese company Biken (Osaka, Japan), which was licensed by the U.S. Food and Drug Administration (FDA) in December 1992 and distributed in the United States by Sanofi Pasteur (176). However, production of JE-VAX was discontinued in 2003, and stockpiles were depleted in 2011. Recognizing this, the inactivated Vero cell–derived vaccine (IXIARO) was licensed by the U.S. FDA in March 2009 for use in adults 17 years of age or older, and in May 2013 for use in children 2 months through 16 years of age; it is distributed by Novartis Vaccines in the United States.
Vaccine Recommendations
In addition to childhood immunizations for persons who live in endemic regions, vaccination against JE virus is recommended for travelers who plan to spend a month or longer in endemic areas during the JE virus transmission season (170,177). JE virus vaccine should be considered for short-term travelers to endemic areas during the JE virus transmission season if they will travel outside of an urban area and their activities will increase the risk of JE virus exposure. JE virus vaccine is not recommended for short-term travelers whose visit will be restricted to urban areas or times outside of a well-defined JE virus transmission season. The dosage and administration is 0.25 mL per dose for children 2 months through 2 years of age, and 0.5 mL per dose for children ≥ 3 years of age and adults. The primary series consists of two doses administered 28 days apart.
POLIO
Neurologic Manifestations of Polio
Nervous System Involvement During Acute Disease
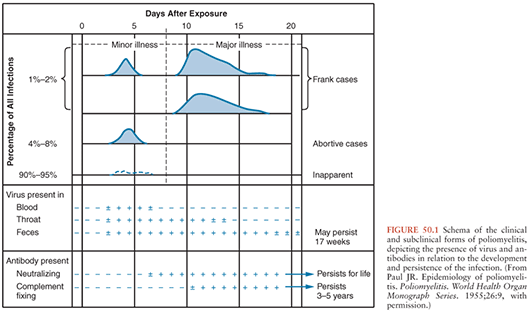
Polioviruses cause three recognizable forms of disease: paralysis, aseptic meningitis, and minor febrile illness. However, most infected individuals will demonstrate no evidence of illness. The clinical and subclinical manifestations of poliovirus infection are depicted graphically in Figure 50.1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree