RICKETTSIOSES, ANAPLASMOSES, AND Q FEVER
DIDIER RAOULT
Bacteria in the order Rickettsiales are gram-negative microorganisms that grow in association with eukaryotic cells. They do not grow in axenic media but require living hosts such as cell cultures, embryonated eggs, or susceptible animals. With the exception of Rickettsia prowazekii, the agent of epidemic typhus, and possibly Rickettsia felis in sub-Saharan Africa, these bacteria infect humans incidentally as zoonoses. On the basis of molecular phylogeny, the bacteria causing rickettsial diseases have been reclassified (Table 27.1). Rickettsioses are emerging infectious diseases, with many new rickettsial diseases having been described in the past 15 years. Rickettsioses can be grouped as follows: Q fever, ehrlichioses, and diseases caused by Rickettsia and Orientia species.
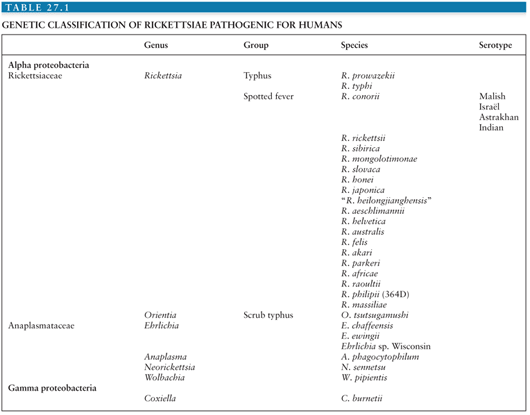
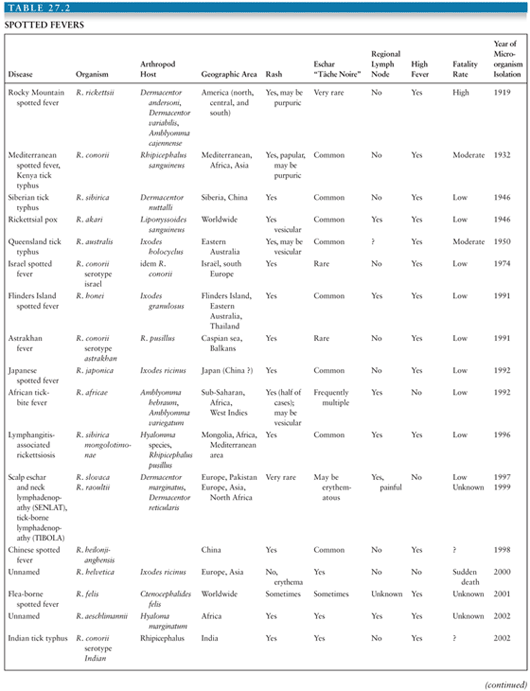
The genus Rickettsia is subdivided into the typhus group, containing Rickettsia typhi and R. prowazekii, and the spotted fever group (SFG) that includes 24 species of organisms pathogenic for humans (Table 27.1). Rickettsiae are associated with arthropods. They are mainly transmitted to humans by bites from infected arthropods, but infections from aerosols of infected insect feces and blood transfusions have also been described (1). Ixodid or hard ticks are the vectors of SFG rickettsiae and have a specific geographic distribution. Mites are the vectors of Rickettsia akari (worldwide) and Orientia tsutsugamushi (linked to Asia), lice are the vectors of R. prowazekii, and fleas are the vectors of R. typhi and perhaps mosquitoes for Rickettsia felis (2) (Table 27.2).
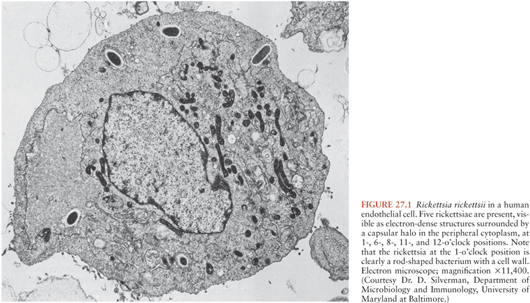
The main clinical signs and symptoms of rickettsioses include fever, headache, a rash that is maculopapular or sometimes petechial or vesicular, inoculation eschars at the site of the arthropod bite, and local lymphadenopathies. Neurologic involvement is mainly associated with severe forms of disease where it is part of a multiple organ dysfunction syndrome (MODS). The main pathologic mechanism in rickettsioses is a vasculitis following infection of the vascular endothelial cells (Fig. 27.1). Apart from the known pathogens, many other rickettsial strains have been found in arthropods, in particular ticks (1), but their roles as human pathogens have yet to be determined.
The first name given to a rickettsial disease, typhus, is indicative of central nervous system (CNS) involvement because it derives from τυποσ (typhos) meaning cerebral confusion. Rickettsioses are seasonal and the arthropod host determines their epidemiology and geographic distribution. Rickettsia species were divided into three taxonomic groups:
• The typhus group comprises R. typhi, the agent of flea-borne, murine typhus, and R. prowazekii causing louse-borne epidemic typhus, Brill-Zinsser disease, and American sylvatic typhus linked to flying squirrels.
• The SFG rickettsiae, which cause tick-transmitted diseases such as Rocky Mountain spotted fever (RMSF) (caused by R. rickettsii), Mediterranean spotted fever (MSF) (Rickettsia conorii), and African tick-bite fever (Rickettsia africae); a mite transmitted disease, rickettsialpox (R. akari); and a spotted fever caused by R. felis that may be transmitted by fleas or mosquitoes (Tables 27.2 and 27.3).
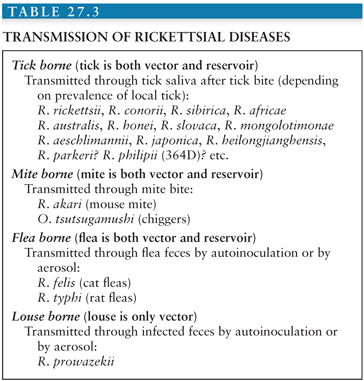
• The scrub typhus group includes O. tsutsugamushi, a mite (chigger)-borne disease.
Coxiella burnetii, the agent of Q fever, belongs to the γ-Proteobacteria phylum. It causes a zoonosis in many mammal species, including domestic animals and wild ungulates, and is excreted in birth products and milk. Humans are usually infected by aerosols and may develop an acute primary infection eventually followed by a chronic disease when predisposing factors are present. Many acute infections cause neurologic signs; meningitis, meningoencephalitis, and Guillain-Barré syndrome have been associated with C. burnetii.
Ehrlichioses and anaplasmoses are zoonoses (3). Ehrlichia species grow in blood cells in a cytoplasmic vacuole, forming clusters or morulae. The bacteria in this large group are increasingly recognized as potential human pathogens, and their current taxonomy has been changed recently to match current phylogenic knowledge. Four genera are described, as follows: One apparently associated with a helminth vector and named Neorickettsia contains only one human pathogen recognized in Japan and Laos (Neorickettsia sennetsu); two are transmitted by ticks, Ehrlichia (including the three human pathogens, Ehrlichia chaffeensis, Ehrlichia ewingii, and Ehrlichia sp. Wisconsin) and Anaplasma (with Anaplasma phagocytophilum being the only currently identified human pathogen). The fourth genus, Wolbachia, has one recognized species, Wolbachia pipientis, which could be associated with arthropods or nematodes. When associated with filaria, in human filariasis, it appears to be a pathogenic factor and a treatment target. The two most common ehrlichioses, the human monocytic ehrlichiosis (HME) caused by E. chaffeensis and human granulocytic anaplasmosis (HGA) caused by A. phagocytophilum may cause neurologic pathology such as meningitis or meningoencephalitis.
RICKETTSIOSES
Historical Background and Emerging Rickettsioses
Epidemic typhus was differentiated from typhoid in the sixteenth century (4) because of the existence of a rash in the rickettsial disease. Some authors suspected an American origin for typhus because of the American sylvatic flying squirrel reservoir. At the beginning of the twentieth century, Ch Nicolle proved the role of lice in typhus transmission, and Ricketts (5) proved that the wood tick, Dermacentor andersoni, was involved in the transmission of RMSF in Montana. In the Old World in 1910, Conor reported the first cases of MSF in Tunis. The role of Rhipicephalus sanguineus was established in 1930 (2).
Recently, several new tick-borne diseases have been identified. Of the rickettsial diseases, only five that are transmitted by ticks were known before 1991 (1), and subsequently, many new diseases have been reported, including Astrakhan (6); Flinders Island spotted fever (7); African tick-bite fever (8); Japanese spotted fever (9); “American Boutonneuse Fever” (Rickettsia parkeri); and, in Europe, infections caused by Rickettsia sibirica mongolotimonae (10), Rickettsia slovaca (11), Rickettsia raoultii, Rickettsia helvetica (12), and Rickettsia aeschlimannii (13) (Table 27.2).
New rickettsial species have frequently been found when arthropod vectors have been studied, that is, ticks, mites, or insects (fleas, lice, etc.). For example, C. burnetii, R. africae, R. conorii caspia, R. felis, R. sibirica mongolotimonae, R. slovaca, R. helvetica, and R. aeschlimannii were first isolated from arthropods and later from people.
Recently, new diseases have been discovered using combinations of isolation, serologic testing, and polymerase chain reaction (PCR) amplification. However, to definitively establish a disease, one should obtain an isolate of the presumptive agent from clinical patients. When this is not possible, an association of serology, immunologic detection of antigens in tissues, and PCR amplification of two different target genes may be used to establish an etiologic relationship between a disease and an organism. Morphologic recognition of rickettsia-like bodies by electron microscopy is of little value (14). Microorganisms found in ticks (15) should be suspected of being human pathogens, because isolates of unknown pathogenicity have erroneously been considered nonpathogenic for years (1).
Diseases
Rocky Mountain Spotted Fever
Epidemiology. RMSF is the most severe of the rickettsioses. It is caused by R. rickettsii and is currently the main tick-transmitted rickettsiosis recognized in America along with R. parkeri (with R. africae in West Indies). It was described first in the nineteenth century in the Western United States but is now known to be prevalent in 44 states in the United States and in Central and South America (Brazil, Argentina, Costa Rica, Columbia, and Mexico).
The main tick vectors are Dermacentor andersoni (the Rocky Mountain spotted wood tick) in Western United States and Dermacentor variabilis (the American dog tick) in the East, the Midwest, and the South. In Central and South America, Amblyomma cajennense is the major identified vector. The duration of attachment is critical for infections, and transmission is unlikely when the tick feeds for less than 20 hours. The tick bite is painless and frequently goes unnoticed. The epidemiology of RMSF undergoes yearly variations that are largely unexplained but probably mainly due to variations in tick activity and human encounters with ticks. There are 500 to 1,000 cases annually in the United States, with 90% reported from April to September, during late spring and summer. The disease is relatively more prevalent in children younger than 10 years.
Clinical Manifestations. The incubation period of RMSF ranges from 2 to 14 days. Initially, patients have a sudden onset of fever and headache, nonspecific signs with a broad list of possible differential diagnoses. This has led to the recommendation that in areas endemic for RMSF, primary care physicians should treat individuals presenting with unexplained fever, with or without other manifestations of RMSF, as if they had the disease (16,17). A rash usually appears 3 to 5 days after the onset of symptoms but is not seen in 10% of patients, especially in dark-skinned patients. It is diffuse and involves palms and soles; it is maculopapular and can be purpuric. Skin necrosis or gangrene involving the digits or limbs may be observed in severe cases (18). Renal failure is common in severe cases (19) and is related to acute tubular necrosis or hypovolemia. Pulmonary manifestations of RMSF range from cough to respiratory distress from pulmonary edema. Retinal abnormalities seen on funduscopic examination include venous engorgement, retinal edema, hemorrhages, papilledema, and arterial occlusion.
Abnormalities in routine laboratory screens are nonspecific; the white blood cell (WBC) count is generally normal, anemia is uncommon, but thrombocytopenia is observed in one third to one half of patients. Hyponatremia and elevations in serum transaminases (e.g., aspartate aminotransferase [AST]) occur commonly.
Analysis of fatal cases of RMSF has shown that older age, hospitalization, and lack of treatment or treatment with chloramphenicol are significantly correlated with death. Treatment with tetracyclines, however, lowered the mortality rate (20). Glucose-6-phosphatase dehydrogenase (G6PD) deficiency is a risk factor for fulminant disease (21).
Neurologic Involvement. The frequency and the severity of neurologic abnormalities depend on the severity of the illness (22). Neurologic complications are often the cause of death. Headache is often severe and diffuse or bifrontal (23–28) (Table 27.4). In one study, 23% of patients had serious CNS complications, including stupor, seizures, delirium, ataxia, focal neurologic deficits, papilledema, and coma (19). Coma is more likely to occur in fatal cases (28). Cranial and peripheral nerve abnormalities can occur, with hearing loss being the most common. Neck stiffness, which is common, is usually related to neck muscle myalgia. The incidence of meningeal signs is about 20% and a diagnosis of bacterial or viral meningitis is often considered (27) (Table 27.5). It was found that 21 (66%) of 32 patients with RMSF who had undergone a lumbar puncture had abnormalities of the cerebrospinal fluid (CSF) and abnormal CSF findings have now been documented in 63 patients (19). There was a pleocytosis in 38%, the protein concentration was increased in 35%, and there was a moderate decrease in glucose concentration in 8%. The WBC count in the CSF is rarely more than 100 cells/mm3. Usually lymphocytes predominate.
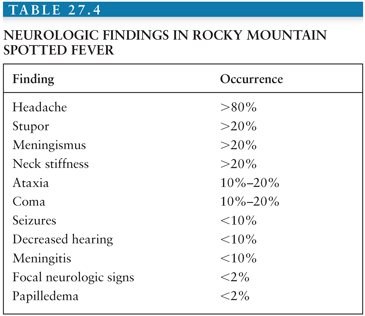
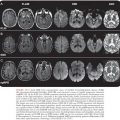
A wide variety of focal neurologic deficits have been described including cranial nerve palsies, aphasia, and ataxia, hemiplegia, complete paralysis, spasticity, and neurogenic bladder (29). Patients with encephalitis may have Rocky Mountain spotless fever (30) and two patients have been described who developed a rash only after a brain biopsy was obtained (22). Therefore, in endemic areas, doxycycline may be added to acyclovir in the treatment of encephalitis. There is very little information on computed tomography (CT) findings in RMSF, but low-density areas and edema have been described (29). The electroencephalogram (EEG) usually shows diffuse cortical dysfunction, especially in comatose patients (19).
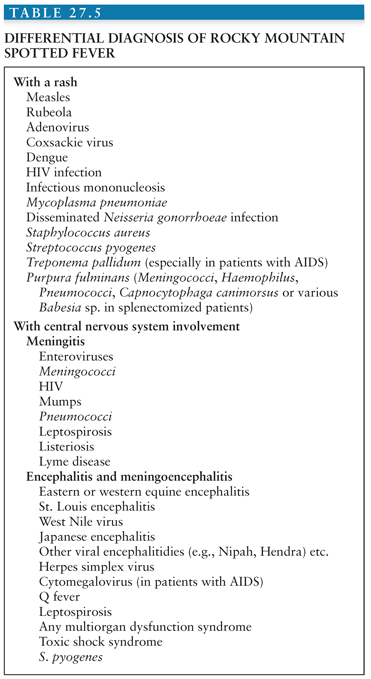
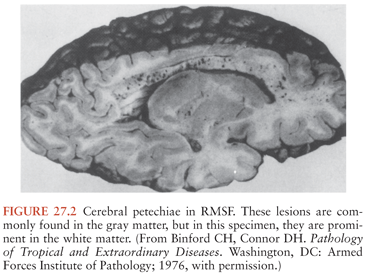
The gross pathologic findings in RMSF involving the CNS include edema and hemorrhage (Fig. 27.2). Microscopically, perivascular accumulations of inflammatory cells, glial nodules, and arteriolar thrombonecrosis with small infarcts have been seen. Microorganisms are readily demonstrated in the endothelial cells of blood vessels using either immunofluorescence (31) or immunoperoxidase techniques (Fig. 27.3).
Residual neurologic deficits are common following RMSF. They include learning disabilities, deafness, behavioral disturbance, depression, transverse myelitis, aphasia, and impairment of fine motor skills (20,32–35). It was reported in one series that 1 to 8 years after recovery, 57% of patients had neurologic abnormalities, including headaches, mild defects in intellectual functioning, and EEG abnormalities (34).
In America, it was believed that RMSF for decades was the only tick-borne rickettsiosis, but a discrepancy appears in the increasing number of reported cases and a lowering fatality rate (35). Because several tick-borne SF were identified such as R. parkeri that causes a mild disease with an inoculation eschar and a rash, R. massiliae infecting Rhipicephalus ticks has been found to cause spotted fever in Europe, R. amblyommii has been linked to fever and rash in Southeastern United States, and a spotted fever with inoculation eschar in California was linked to a new rickettsia (Rickettsia philipii 364D). Finally, R. africae is prevalent in West Indies and reported in American travelers to Africa. However, these diseases are generally mild with rare involvement of the CNS.
Other Tick-Borne Rickettsioses
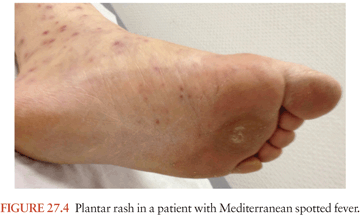
Tick-transmitted rickettsioses have limited geographic distributions that are determined by their tick vectors. In Europe, MSF is caused by R. conorii and is also known as boutonneuse fever, Marseilles fever, Astrakhan fever, Israeli spotted fever, Indian tick typhus, or Kenyan tick typhus. It resembles RMSF but has specific features. The disease is milder, but a fatality rate of 1.5% to 2.5% of hospitalized patients is still found. A malignant form of the disease with purpuric rash, shock, and MODS has been described in alcoholic, diabetic, human immunodeficiency virus (HIV)–infected, and old, or debilitated patients. The typical clinical presentation is that of a patient with fever, a rash (which may involve the palms and soles [Fig. 27.4]), and a “tâche noire,” that is, a black eschar at the site of the tick bites. The “tâche noire” is found in 50% to 80% of patients. The rash is often papular, which led to one of the names of the disease (boutonneuse fever). Israel tick-bite fever and Astrakhan fever appear milder than typical MSF, and a “tâche noire” is often lacking.
R. slovaca and R. raoultii causes a newly described disease that appears to be common in Europe. The tick vectors, Dermacentor marginatus and Dermacentor reticulatus, feed mainly in the cold months and tend to attach on the scalp of people, preferring haired sites. The disease is more prevalent in children and women, contrary to other tick-borne rickettsioses. It is rarely exanthematic, with the typical clinical picture including an erythematous skin lesion ranging from 2 to 8 cm in diameter at the site of the tick bite on the scalp and regional lymphadenopathy (which may be painful). Rarely, patients have fever and a rash, and a deep postinfectious asthenia and residual alopecia at the site of the tick bite can be observed. A case of meningoencephalitis has been reported. The occurrence of this rickettsiosis without rash may stimulate research on other new rickettsial diseases with only localized manifestations (36). This disease has been named successively TIBOLA (for tick-borne disease), DEBONEL (for Dermacentor-borne eschar and lymphadenopathy) and SENLAT (scalp eschar and neck lymphadenopathy after tick bite) (36a).
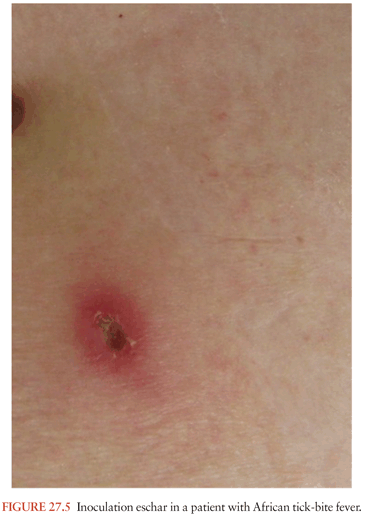
In Africa, African tick-bite fever may be the most common tick-borne rickettsiosis worldwide. It is due to R. africae, which is transmitted by African ticks, Amblyomma hebraeum, and Amblyomma variegatum. They usually feed on ungulates but also feed readily on human beings and cause a high prevalence of infection in rural Africa (60% of tested patients exhibit antibodies) and in travelers. More than 50% of patients have more than one “tâche noire” (Fig. 27.5), which are most often on the lower limbs and are often associated with regional lymphadenopathy in the groin. The disease differs from the other milder rickettsioses in that fever is frequently absent; a rash is present in only half of the patients with the disease; and the rash may be vesicular (which has never been reported in confirmed MSF) in half of the cases. An aphthous stomatitis can be associated with the disease. In Asia, Japanese spotted fever (caused by Rickettsia japonica), Siberian tick typhus (caused by Rickettsia sibirica), and infections caused by R. mongolotimonae comprise commonly and ropelike lymphangitis between the inoculation eschar and a lymph node. Rickettsia australis (Queensland tick typhus) and Rickettsia honei (Flinders Island spotted fever) cause diseases resembling MSF, but their rash can be vesicular. The neurologic involvement of the other tick-borne spotted fevers is comparable to that of RMSF. MSF has been associated with meningitis, meningoencephalitis, deafness, stupor, and coma (often with MODS) (37). Meningoencephalitis was reported in two patients with Japanese spotted fever (38).
The spotted fever caused by R. felis is a new incompletely defined disease. The bacterium is found in fleas in the United States, Peru, Europe, and Africa and in mosquitoes in Africa. It has been grown only recently (39,40). Seven cases have been reported from Texas, Mexico, Brazil, France, and Germany. All had fever, six of seven had a rash, and an inoculation eschar was present in some patients (39,41). A diagnosis can be based on serology using specific R. felis antigen or PCR of blood or skin biopsies. The most effective treatment has not been established, but the bacterium is highly susceptible to doxycycline (42). Recently, it was reported commonly associated with fever in Kenya and Senegal where malaria is endemic, causing as much as 6% of fevers (2a). The infection in children in Africa consists of fever and vesicular rash and was named Yaaf (42a).
Rickettsialpox (Rickettsia akari)
Rickettsialpox was first described in New York City, where it is still prevalent (43). The recent terrorism events (of September 11, 2001) have led physicians to pay increased attention to skin eschars (as might be seen with anthrax) and vesicular rashes (which might be seen with smallpox), and this has led to an increase in the cases of rickettsialpox diagnosed in New York City between 2001 and 2002 (44). R. akari, the causal agent, is an SFG rickettsia transmitted by the bite of the mouse mite (Liponyssoides sanguineus). Serologically, it cross-reacts with other SFG rickettsia. The prevalence of infections is probably underestimated, although cases have also been reported from Arizona, Utah, and Ohio and a high seroprevalence was found in intravenous drug users in Baltimore. Cases have also been diagnosed in Russia, Ukraine, Slovenia, and Korea (1). Ten days after the mite bite, the beginning of the illness is marked by fever, headache, and myalgia. A careful examination will reveal an inoculation eschar and regional lymphadenopathy. Two to six days later, a rash appears consisting of 5 to 40 macular then papular to vesicular (or even pustular) lesions. The name of the disease was derived from the latter, and it is often mistaken for chickenpox. The disease is usually mild (1).
Epidemic Typhus
Typhus is transmitted by the human body louse, which lives in clothes and multiplies rapidly when cold weather and lack of hygiene allow it to. Its prevalence reflects the low socioeconomic status of certain members of a society (45) and rises during war, in poor countries in refugee camps, and in homeless people in rich countries, including the United States and Europe. Recent reports of cases have been made in Burundi (46), Rwanda, Russia, Peru, the United States, and Algeria (47).
Humans are the reservoirs of R. prowazekii and lice are the vectors. They are infected by ingesting R. prowazekii in a blood meal. The organism multiplies in the gut and is released in feces (48), where R. prowazekii can survive for weeks. Patients are infected by aerosols or by inoculation of infected feces into the skin during scratching. Patients who recover from typhus may have latent infections and relapse years later with stress. The relapsing form is named Brill-Zinsser disease and is associated with bacteremia that might lead to lice becoming infected and the start of a new outbreak (1). In the United States, the eastern flying squirrel (Glaucomys volans) and its fleas, lice, and mites can be infected. They constitute a sylvatic reservoir and generate cases of domestic typhus (49).
Typhus begins abruptly with fever, headache, and myalgias. Coughing is also common, as is neurologic involvement, evidenced by stupor, confusion, or coma (Figs. 27.6 to 27.8). A rash is observed in 20% to 80% of patients but may be difficult to observe in dark-skinned people. It usually starts in the axilla and then spreads. It is usually macular but may be purpuric in severe cases. Diarrhea and jaundice are often reported. Splenomegaly is found only infrequently. In severe cases, shock is observed and the spontaneous fatality rate is 20% to 30%. Abnormal laboratory results might include leukopenia, thrombocytopenia, anemia, and increased serum hepatic enzymes. The disease should be considered in patients with high fever and confusion and in patients exposed to lice. In tropical countries, epidemic typhus might be confused with typhoid, malaria (50), hemorrhagic fevers, and dengue. This could have fatal consequences because the prescribed treatments for typhoid (β-lactams, co-trimoxazole, and quinolones) are ineffective in typhus. In people with lice, it can be confused with trench fever and relapsing fever, in which the same treatment can be prescribed (46).
Brill-Zinsser disease is the late relapsing form of typhus that is frequently undiagnosed as a rash and recent exposure to lice are commonly not present (51). Interviewing the patient may reveal previous exposure to lice, associated, or not, with a previous diagnosis of typhus. The disease is mild and the prognosis is good.
Sylvatic typhus in the United States is caused by an R. prowazekii variant and is a milder disease. The most prominent clinical features are neurologic, with meningitis being the clinical manifestation (49). Few cases have been described and nearly all occurred in areas east of the Mississippi, where the eastern flying squirrel is found. In these areas, cases may be observed in winter and sylvatic typhus should be considered in patients with a rash.
Murine Typhus
Murine typhus is associated with rat and opossum fleas. Humans are infected when contaminated flea feces are inoculated into the skin during scratching at the sites of flea bites. The disease is more prevalent in hot and humid areas, specifically when rats proliferate. In the United States, 50 to 100 cases are reported yearly, mainly in southern California and southern Texas. Recently, cases have been described in Mexico, Indonesia, Southern Europe, and Africa (52,53).
The incubation period ranges from 8 to 16 days. The disease begins abruptly with fever, myalgias, arthralgias, nausea, vomiting, and headache. A discrete rash is observed in 40% to 50% of patients, on average, 6 days after the onset of signs. It is detected less often in dark-skinned patients. The rash usually consists of pink macules but may become maculopapular (54). It begins in the axilla and generalizes to the trunk but usually does not involve the face, palms, and soles. It can become purpuric in severe cases. One third of patients have a cough and one fourth of the patients have nonspecific interstitial pneumonia sometimes associated with pleural effusion. In severe forms, respiratory distress might require intubation and artificial ventilation. Neurologic symptoms range from confusion and stupor to coma and seizures in severe forms. Cerebral hemorrhages may occur. Digestive involvement can manifest as vomiting, abdominal pain, jaundice, and in severe cases, hematemesis (52,55).
Laboratory abnormalities include leukopenia, which may be followed by leukocytosis. There might also be thrombocytopenia and anemia, especially when hemolysis is observed (often in patients with G6PD deficiency). A moderate increase in serum liver enzymes is common. In patients with severe disease, hyponatremia and hypoalbuminemia are observed.
The prognosis is usually favorable, but 10% of patients require intensive care and 1% die. The neurologic complications of murine typhus usually manifest during the second week of illness (56). Aseptic meningitis occurs in 2% to 5% of patients (56). Meningoencephalitis, which is rare, occurs in older patients. Such patients usually have seizures. Papilledema rarely occurs (57). The CSF WBC count rarely exceeds 150 cells/mm3 and mononuclear cells predominate (56).
Scrub Typhus (Orientia tsutsugamushi)
Scrub typhus is transmitted by the bite of trombiculid mite larvae infected by O. tsutsugamushi. It is prevalent in a triangle extending between northern Japan, eastern Australia, and eastern Russia, and including the Far East, China, and the Indian subcontinent. Altogether, 1 billion people may be exposed. Seasonality is determined by emergence of larvae. In temperate zones, it occurs mainly in autumn and to a lesser extent in spring. O. tsutsugamushi species have a wide heterogenicity. The more common serotypes are Kato, Karp, Gilliam, and Kawasaki (58).
The incubation period in rural or urban scrub typhus is 10 days or more. The onset of signs is usually sudden and associated with fever, headache, and myalgias. With careful examination, an inoculation eschar may be found at the site of the mite bite and draining lymph nodes may be tender. Generalized lymphadenopathies and rash may be observed. The symptoms vary according to organ involvement. Neuromeningeal symptoms are relatively common. Severe forms can occur, associated with septic shock.
The fatality rate in untreated patients is 6% to 10%. In a study of scrub typhus in Thailand, 9 (13%) of 72 patients presented with meningitis or encephalitis syndromes, or both (59). One of the nine had cerebellitis and another had papilledema (59). Focal neurologic signs may predominate (60). The CSF contains mainly mononuclear WBCs (59). Laboratory abnormalities may include leukopenia, thrombocytopenia, and increased levels of hepatic enzymes. Interestingly, scrub typhus is not more severe in HIV-infected patients and an HIV-suppressive factor appears to be produced during infection. Relapses of the disease may occur (61,62). Diagnosis may be difficult because the clinical presentation is often nonspecific and identifying epidemiologic factors is critical. Infectious mononucleosis is commonly confused with scrub typhus.
Diagnostics
Serology
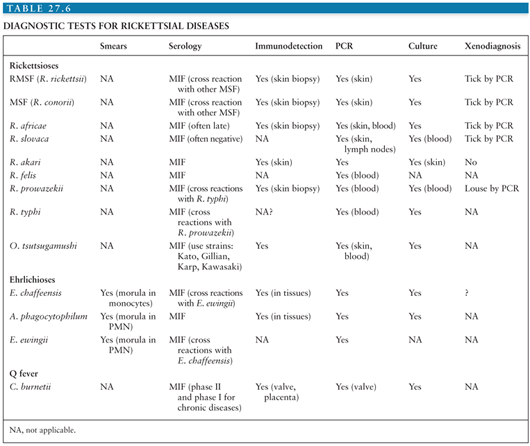
Serologic assays are the simplest diagnostic tests to perform (Table 27.6). The Weil-Felix test was the first to be used and involves antigens from three Proteus strains: Proteus vulgaris OX-2, P. vulgaris OX-19, and Proteus mirabilis OXK. It was used widely to detect rickettsioses based on serologic cross reactions (63), but the test lacks sensitivity and specificity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree