MENINGITIS AND ENCEPHALITIS CAUSED BY MUMPS VIRUS
JOHN W. GNANN, JR.
Mumps is an acute systemic human infection caused by a paramyxovirus. Mumps virus is highly transmissible and, in unimmunized populations, causes epidemics of mumps among school-aged children. Although salivary gland enlargement, especially parotitis, is the most readily recognized clinical manifestation of mumps, the infection involves many other organs, including the central nervous system (CNS). In the past, nonsuppurative parotitis was considered the sine qua non of mumps, and involvement of any other organ system was viewed as a complication. However, studies conducted over the last 60 years have made it clear that CNS involvement during mumps occurs with such high frequency that it should be considered a part of the natural history of the infection and not as an aberrant manifestation or complication. Indeed, some virologists have classified mumps virus as primarily neurotropic.
The spectrum of CNS diseases associated with mumps ranges from mild aseptic meningitis, which is very common, to fulminant and potentially fatal encephalitis, which is very rare. The literature describing CNS involvement with mumps can be difficult to assess critically because some authors have grouped all cases of mumps neurologic disease under the label “mumps meningoencephalitis.” Although this term is convenient, its use obscures the fact that the clinical course and prognosis of mumps aseptic meningitis differs markedly from that of mumps encephalitis, although there is considerable overlap between the two syndromes. It is important for the clinician to establish whether an individual patient with mumps has clear evidence of encephalitis because that diagnosis has important implications for management and prognosis.
EPIDEMIOLOGY
In unvaccinated urban populations, mumps (or “epidemic parotitis”) is a disease of school-aged children with a worldwide distribution. Mumps infrequently occurs in infants younger than 1 year of age, presumably because of transplacentally acquired antibody. Most mumps cases occur in children between 4 and 7 years of age (1). By age 15 years, over 90% of children have antibodies against mumps virus.
Prior to the release of the live attenuated mumps vaccine in the United States in 1967, mumps was an endemic disease with a seasonal peak of activity occurring between January and May (2). The largest number of cases reported in the United States occurred in 1941, when the incidence of mumps was 250 cases per 100,000 population (3). In 1968, when the live attenuated vaccine was first being put to clinical use, the incidence of mumps was 76 cases per 100,000 population. In 1985, a total of only 2,982 cases of mumps were reported, an incidence of 1.1 cases per 100,000 population, representing a 98% decline from the 185,691 cases reported in 1967 (4). Sporadic outbreaks of mumps in secondary schools occurring in the United States were attributed to primary vaccine failure (5). Between 1988 and 1993, 36% of all mumps cases in the United States were in patients older than 15 years of age (6). In 1989, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended that all children receive a second dose of the measles-mumps-rubella (MMR) vaccine at the time of school entry, which reduced the rate of primary vaccine failure (4). The incidence of mumps in the United States declined steadily after 1987; only 387 cases were reported in 1999 (7). By testing sera from the 1999 to 2004 U.S. National Health and Nutrition Examination Survey (NHANES), the overall age-adjusted seroprevalence of immunoglobulin G (IgG) to mumps virus was shown to be 90% (8).
However, a series of outbreaks in the United States and Canada in 2005 to 2006 reemphasized the potential for mumps virus to cause localized epidemics even in highly vaccinated populations (9,10). In 2006, a total of 6,584 cases of mumps were reported, with a national incidence of 2.2 cases per 100,000 population (9). The U.S. outbreak was centered in the Midwest and peaked in April 2006; 34% of the cases occurred in Iowa. The highest age-specific age rate was in persons aged 18 to 24 years (median age 22 years), many of whom were college students (11). Among the Iowa cases, 7% were unvaccinated; 14% had received one dose of MMR; 49% had received two or more doses of MMR; and 30% had unknown vaccine status. Although some cases can be attributed to failure to vaccinate, most represented primary (insufficient initial immune response) or secondary (waning immune response) vaccine failure (12). These findings suggest the need for a mumps vaccine with a longer duration of protection or modification in vaccination policy, perhaps focusing on young adults (9,13). In selected outbreaks, a third dose of mumps vaccine has been administered for outbreak control, which appears to be a safe and effective measure (14,15).
The importance of immunization of adolescents and young adults was emphasized by a large outbreak (over 56,000 reported cases) of mumps in the United Kingdom in 2004 to 2005 (16,17). Many of the cases occurred in university students who were too old to have been vaccinated in childhood but too young to have been exposed to epidemic natural infection (18). Conversely, a large mumps outbreak (>3,500 cases) occurred in 2009 to 2010 in New York and New Jersey, causing disease primarily among adolescent boys attending Orthodox Jewish schools (19). Mumps transmission in this highly vaccinated population was apparently facilitated by the close face-to-face interactions among the students. The propensity for mumps to spread among vaccinated adolescents in schools and communal living environments raises the question of whether a revised vaccination schedule should be considered (20–22).
In unimmunized populations, mumps is one of the most common causes of aseptic meningitis and encephalitis (23). In a survey conducted in Minnesota from 1950 to 1981, mumps was identified as the cause of encephalitis in 6 of 189 cases and the cause of aseptic meningitis in 7 of 283 cases (24). In that series, mumps was the second most common cause of both encephalitis and aseptic meningitis (behind California virus and enteroviruses, respectively). Meyer et al. (25) reviewed 713 cases of encephalitis and aseptic meningitis treated at U.S. military hospitals between 1953 and 1958. In those patients in whom a specific etiology was established, mumps was identified in 91 cases, poliomyelitis in 156, coxsackievirus B in 80, lymphocytic choriomeningitis virus in 58, and echovirus in 53. In an analysis of 191 cases of acute encephalitis occurring in adults in Finland between 1967 and 1978, mumps virus was found to be the second most common etiologic agent (following herpes simplex virus), accounting for 6.8% of the cases (26).
The frequency with which CNS manifestations occur during acute mumps has varied tremendously (from <1% to >70%) among published series (27,28). A more realistic estimate of the frequency of symptomatic CNS involvement during mumps appears to be 10% to 30%. Potential causes for this variability in reported incidence include the interest and skill of the observer, the population studied (e.g., schoolchildren vs. military recruits, outpatients vs. hospitalized patients), the case definitions used, and the frequency of use of lumbar puncture for diagnosis. Symptomatic encephalitis is observed much less frequently than aseptic meningitis, probably occurring in fewer than 0.1% of cases of acute mumps (29–31). Cerebrospinal fluid (CSF) pleocytosis occurs in 40% to 60% of patients with acute mumps, although only 10% to 30% of patients with mumps have clinical evidence of meningeal irritation. That is, about half of the patients with mumps with CSF pleocytosis do not have CNS symptoms (28,32). Bang and Bang (27) performed lumbar punctures on 371 patients with mumps parotitis and found that 235 (63%) had elevated CSF white blood cell counts; of these 235 patients, 129 showed no clinical evidence of meningitis or encephalitis. Similarly, Finkelstein (33) found elevated CSF white blood cell counts in 16 of 40 patients (40%) with mumps parotitis; 6 of these had no clinical evidence of CNS involvement. Clearly, mumps CNS disease can also occur in patients without evidence of parotitis; indeed, 40% to 50% of patients with mumps meningitis have no evidence of salivary gland enlargement (34,35). If we accept that CSF pleocytosis occurring during acute mumps is indicative of CNS infection, then we must conclude that CNS involvement during mumps is quite common and frequently asymptomatic.
Although male and female patients have the same risk for mumps parotitis, there is a distinct male predominance (70% to 80%) with respect to development of CNS disease among children with mumps. In virtually every published series, the ratio of boys to girls is between 3:1 and 4:1 (28,30–32,36–39). This striking difference in the incidence of mumps CNS disease between the sexes has not been satisfactorily explained. Among young adults with mumps and CNS involvement, the ratio of men to women is closer to 1:1 (39). The peak incidence of CNS involvement in mumps occurs at about age 7 years in both sexes (31,39,40). Of all cases, 60% to 70% occur in children between 5 and 9 years of age (28,30,37,38,41). Several authors have reported seasonal differences in the frequency of mumps meningoencephalitis, with and without parotitis (34,35,40–44).
INFECTIOUS AGENT
Mumps virus is classified as a member of the family Paramyxoviridae and the genus Rubulavirus (45). In general, members of the family Paramyxoviridae possess nonsegmented RNA genomes, bind to neuraminic acid receptors on host cells, replicate in the cytoplasm, are inactivated by ether, and cause respiratory infections in humans (46). Serologic testing with hemagglutination inhibition (HI) or neutralization assays has identified only one serotype of mumps virus, although minor antigenic differences among mumps virus isolates can be detected using panels of glycoprotein-specific monoclonal antibodies (47). Infection can be prevented by vaccination with a single strain of mumps virus. Mumps virus shares some serologic cross reactivity with other paramyxoviruses, especially parainfluenza viruses, which can complicate interpretation of serodiagnostic assays (48).
Mumps virions are pleomorphic, irregularly spherical, enveloped particles with an average diameter of about 200 nm. Glycoprotein spikes project from the outer surface of the lipid envelope, which encloses a helical nucleocapsid composed of RNA and nucleoproteins. The mumps virus genome consists of a linear molecule of single-stranded, negative-sense RNA approximately 15.3 kilobases in size that encodes seven major proteins and several minor proteins. The three proteins contained in the ribonucleoprotein complex are the nucleocapsid protein (NP), which is the major structural protein of mumps virus, plus the phosphoprotein (P) and large (L) protein, which are thought to function as the RNA-dependent RNA polymerase (49). Full-length transcription of the V/P gene yields the V protein (also known as NS1), whereas RNA editing during the transcription of V/P produces the phosphoprotein as well as the nonstructural I protein (also known as NS2) (50). The V protein blocks host antiviral responses by inhibiting the interferon signal transduction pathway and interleukin (IL)-6 expression and may serve as a virulence factor (51–53). The function of the I protein is not known. The envelope contains the matrix (M) protein and the two surface glycoproteins that mediate hemagglutinin-neuraminidase (HN) and fusion (F) activities. The HN glycoprotein is responsible for attachment of mumps virus to the receptors on the host cell, and the F glycoprotein induces the fusion of lipid membranes necessary for penetration of the virus nucleocapsid into the cell. In traditional complement fixation (CF) assays, HN and NP are known as “V antigen” and “S antigen,” respectively.
The function of the membrane-associated protein encoded by the small hydrophobic (SH) gene has not been fully defined, although it does not appear to be essential for viral replication (54). Characterization of the genetically divergent SH region is a useful tool for distinguishing wild type and vaccine strains of mumps virus (55). Furthermore, studies employing reverse transcriptase polymerase chain reaction (RT-PCR) methodology have demonstrated 13 distinct viral genotypes (designated A to M) based on sequence of the SH gene (56–58). These molecular tools will allow more precise studies of mumps transmission and population genetics (59–62). In the Western hemisphere, genotypes A, C, D, E, and H are more common, whereas genotypes B, F, and I predominate in Asia. Although there is only a single mumps serotype, some investigators have found that cross-neutralization between mumps virus genotypes is reduced (63,64). Some studies have suggested that infection with certain genotypes (e.g., C, D, H, or J) may result in enhanced neuropathogenicity (60,65), but this observation was not confirmed by other investigators (58).
Humans are the only known natural hosts for mumps virus, although infection can be experimentally induced in a variety of mammalian species. In vitro, mumps virus can be cultured in many mammalian cell lines, including primary Rhesus monkey kidney, human embryonic kidney, BSC-1, Vero, and HeLa cells, as well as in embryonated hens’ eggs (66). In tissue culture, the cytopathic effects caused by mumps virus appear within 3 to 6 days and are highly variable. They include development of rounded cells, formation of eosinophilic intracytoplasmic inclusions, and fusion of cells to form giant multinucleated syncytia. Guinea pig or chick erythrocytes will adhere to mumps virus–infected cells, a phenomenon mediated by hemagglutinins present on the surface of infected cells. Traditionally, verification of the isolate as mumps virus has been accomplished by a hemadsorption inhibition assay, in which mumps virus–specific antiserum is used to block the adherence of erythrocytes to mumps-infected cells. Most laboratories now use direct or indirect immunofluorescence methods to identify mumps virus in tissue culture (66). Molecular methods based on PCR have also been described (67).
PATHOGENESIS AND PATHOPHYSIOLOGY
Mumps is highly contagious, although some studies have suggested that it is less contagious than varicella or measles (68). This clinical observation may be skewed by the fact that up to 30% of all mumps infections are subclinical and asymptomatic (69). Over 90% of adults who give negative histories for mumps are, in fact, seropositive when tested for mumps antibodies, indicating prior subclinical infection (70).
Mumps can be experimentally transmitted to humans by inoculation of virus onto the nasal or buccal mucosa, suggesting that most natural infections result from droplet spread of upper respiratory secretions from infected to susceptible individuals. Virus can be isolated from saliva for 5 to 7 days before and 5 to 8 days after the onset of clinical symptoms, meaning that an infected individual is potentially able to transmit mumps for a period of up to 2 weeks (71,72). The average incubation period for mumps is about 18 days (range 14 to 28 days) (73,74). During this interval, primary viral replication is thought to take place in epithelial cells of the upper respiratory tract, followed by spread of virus to regional lymph nodes and then viremic dissemination to glandular and neural tissue (75,76).
Mumps virus is delivered to the CNS either via free plasma viremia or by infected host mononuclear cells (77,78). Virus is thought to spread across the endothelium of the choroid plexus and to infect choroidal epithelial cells. Replication of mumps virus then takes place in the choroidal epithelium, and progeny virus is shed into the CSF. In support of this model is the observation that mumps virus can be easily recovered from CSF during the early phases of mumps meningitis in humans. Additionally, choroidal and ependymal epithelial cells containing mumps antigens can be recovered from the CSF of patients with mumps meningoencephalitis (79). Replication of mumps virus in the choroid plexus and ependyma (the tissue that lines the cerebral ventricles and covers the choroid plexus) has been demonstrated in rodent (77) and primate (80) models of mumps CNS infection. When mumps encephalitis develops, it is presumed that virus replicating in ependymal cells spreads by direct extension into neurons within the brain parenchyma, as has been observed in the hamster model of mumps encephalitis (81).
PATHOLOGY OF MUMPS CENTRAL NERVOUS SYSTEM INFECTION
Autopsy reports of patients who have died with acute encephalitis caused by mumps virus have been reviewed by Donohue (82) and Schwarz et al. (83). Many of these cases occurred prior to the availability of modern virologic or serologic methods for confirmation of the diagnosis, and some were likely caused by pathogens other than mumps virus. Among those cases in which mumps virus was the probable cause of the fatal encephalitis, neuropathological findings were quite variable (28,82–84). The most commonly recorded features were diffuse edema of the brain, limited mononuclear cell infiltration of the meninges, perivascular infiltration with mononuclear cells, glial cell proliferation, focal areas of neuronal cell destruction, and localized demyelinization. These histologic changes were seen in the white matter of the cerebral hemispheres and cerebellum and in the white and gray matter of the brainstem and spinal cord. Because of the pattern of perivascular demyelinization, Donohue (82) and Schwarz et al. (83) suggested that these findings were more suggestive of a postinfectious or parainfectious encephalopathy rather than of tissue destruction caused directly by mumps virus. Conversely, other authors have interpreted the findings of cellular destruction as consistent with primary mumps virus cytopathic effect (85). In most cases of fatal mumps encephalitis that have been carefully studied, there has been histologic evidence of both cellular destruction (suggestive of direct virus effect) and demyelinization (suggestive of an autoimmune process). The pathogenesis of mumps encephalitis remains incompletely understood.
Investigations using the hamster model have made major contributions to our understanding of the pathogenesis of mumps CNS disease. CNS mumps infections have been produced experimentally by the intracerebral or intraperitoneal inoculation of newborn hamsters with a neuroadapted strain of mumps virus (77,86). About 9 to 12 days after intracerebral inoculation with mumps virus, suckling hamsters developed wasting, ruffled hair, and arched backs; they usually died within 24 hours. Maximum titers of mumps virus were detected in hamster brains at 5 days, and the virus titer declined as neutralizing antibodies appeared. The clinical symptoms occurred 3 to 5 days after the decline in virus titers and the concurrent development of neutralizing antibody (86). During the first week after virus inoculation, the neuropathologic appearance of the brain was characterized by intense perivascular mononuclear cell infiltrates. During the second week, microglial cell proliferation and small areas of necrosis were observed. Notably, no foci of demyelinization (as has been observed in human brains) were apparent.
Immunofluorescence studies of suckling hamster brains following intracerebral inoculation with mumps virus showed that mumps virus antigens were present in endothelial cells of the choroid plexus, in ependymal cells, and in neurons of the brainstem, hippocampus, and cerebral cortex. Most of the infected neurons were morphologically normal, and there was little correlation between the major sites of neuronal infection and the observed sites of perivascular inflammation (86). The histologic appearance of mumps encephalitis in suckling hamsters is more consistent with direct virus-induced pathology than with immunologically mediated pathologic changes. Studies using the suckling hamster model have also demonstrated an association of mumps CNS infection with the development of stenosis of the aqueduct of Sylvius and with granular ependymitis (87). These findings suggest a possible (but unproven) linkage between mumps CNS infections and aqueductal stenosis in children (88,89).
The ability of mumps virus to establish chronic infections in tissue culture systems is well known (90,91). In the hamster model, cell-associated mumps virus can be recovered from brain explant cultures for up to 50 days after infection, well after the appearance of specific humoral immunity (77). In humans with mumps meningoencephalitis, the persistence of leukocytes and oligoclonal mumps-specific immunoglobulins in the CSF for months after the acute infection suggests the possibility of ongoing antigenic stimulation from chronic mumps CNS infection (92,93). Sufficient data do not yet exist to confirm or refute a potential for mumps virus as a cause of chronic CNS infection in humans, but chronic infection, if it occurs at all, is uncommon (92,94).
IMMUNE RESPONSES
Specific humoral and cell-mediated immune responses develop during the course of acute mumps infection, but the relative contributions of antibody and cellular immunity to viral clearance have not been precisely determined. Interestingly, mumps does not often cause unusually severe or prolonged infections in immunocompromised patients, although severe nephritis due to mumps virus has been reported in renal transplant recipients (95).
Within 10 days of infection, mumps-specific immunoglobulin M (IgM) appears in serum and immunoglobulin A (IgA) can be detected in saliva (96,97). Serum IgM titers begin to wane shortly after the acute illness and are usually undetectable after 6 months (96,98). A mumps-specific IgG response is detectable during the first week of the acute infection, peaks about 3 to 4 weeks after the onset of the infection, and persists for decades (99). Using the CF assay, a fourfold rise in mumps-specific IgG can usually be demonstrated within 10 to 14 days after the onset of the disease (100). Lifelong immunity follows natural infection. Symptomatic mumps virus reinfections have been reported, but most patients who report more than one episode of mumps probably had parotitis caused by infection with a different pathogen (101).
Mumps-specific immunoglobulins are also detectable in CSF of patients with mumps meningoencephalitis (102). Using a sensitive enzyme-linked immunoadsorbent assay (ELISA) method, mumps-specific IgG was detected in almost all CSF specimens from patients with mumps meningitis, and mumps virus IgM was detected in about 50% of patients (103). By measuring the levels of immunoglobulin in serum and CSF and then comparing this ratio with that of an index antibody (such as measles antibody), intrathecal synthesis of mumps-specific immunoglobulin was shown to occur during mumps CNS infection. There was no apparent correlation between the severity of clinical disease and the presence or absence of mumps IgM in the CSF (104). Furthermore, no correlation between the CSF leukocyte count and the CSF mumps antibody titer was demonstrated (104). Infection (or vaccination) with mumps virus also elicits a cell-mediated immune response (105). Peripheral blood lymphocytes that proliferate when stimulated with mumps S and V antigens can be detected by an in vitro blastogenesis assay following natural infection or immunization (106). Lymphocytes recovered from the CSF of patients with mumps meningitis also proliferate when stimulated with mumps antigens (107). Human leukocyte antigen (HLA)–restricted cytotoxic T lymphocytes (CTLs) are detectable in peripheral blood following mumps infection or immunization and in CSF of patients with mumps CNS infection (108). Kreth et al. (109) demonstrated that T lymphocytes from CSF and from blood from 10 children with mumps meningitis were cytotoxic to autologous mumps virus–infected target cells. Lymphocyte-mediated toxicity was present during the acute phase of mumps meningitis, declined over 2 to 3 weeks after the onset of symptoms, and was no longer apparent after 50 days. Fleischer and Kreth (110) cloned mononuclear cells directly from the CSF of a patient with mumps meningitis and demonstrated that 90% of the cells were T lymphocytes and that 60% were CD8+ suppressor/ cytotoxic T cells. A high percentage of the T-cell clones showed specificity for the autologous mumps virus–infected target cells. In patients with self-limited mumps meningitis, CSF interferon disappeared within a week, whereas interferon levels remained elevated in the CSF from those patients who had persistent CSF pleocytosis (111). These findings suggest that the recruitment of CTLs into the CNS in mumps meningitis is highly antigen specific and that mumps-specific CTLs could play a role in the immunopathologic changes observed in human brains after fatal mumps encephalitis. Recent studies have also demonstrated increased levels of IL-8, IL-10, IL-12, IL-13, and interferon (IFN)-gamma in CSF from children with mumps meningitis (112).
CLINICAL COURSE AND NATURAL HISTORY
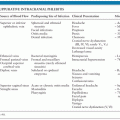
Mumps usually begins with a short prodromal phase characterized by low-grade fever, malaise, headache, and anorexia. Young children may initially complain of ear pain. The patient then develops the characteristic salivary gland enlargement and tenderness (113). The parotid glands are most commonly involved, although other salivary glands (e.g., submandibular and sublingual) may be enlarged in about 10% of cases. Parotitis may initially be unilateral, with swelling of the contralateral parotid gland occurring 2 to 3 days later; bilateral parotitis eventually develops in over 80% of patients with symptomatic salivary gland involvement. Painful parotid gland enlargement progresses over the course of 2 to 3 days, lifting the earlobe outward and obscuring the angle of the mandible (Fig. 16.1). The orifice of Stensen duct is often red and edematous. Lymphatic obstruction resulting from bilateral glandular enlargement can occasionally result in presternal edema (114). Fever and parotid gland swelling peak on about the third day of the illness, followed by defervescence and resolution of parotid pain and swelling within about 7 days. Long-term sequelae of parotitis are uncommon. Children with mumps are usually isolated for about 5 days after the appearance of parotitis, although this practice is of dubious benefit to classmates, because the virus is excreted for several days prior to the onset of clinical symptoms (115).
Epididymoorchitis is rare in boys with mumps, but it occurs in 25% of postpubertal men with mumps infection (19,116,117). Orchitis is caused by replication of mumps virus in seminiferous tubules, with resulting lymphocytic infiltration and edema (118–120). Orchitis is most often unilateral, but bilateral involvement occurs in 10% to 30% of cases (121). Orchitis typically develops within 1 week after the onset of parotitis, although orchitis can develop prior to or even in the absence of parotitis. Mumps orchitis is characterized by marked testicular swelling and severe pain, accompanied by fever, nausea, and headache (121–123). The pain and swelling resolve within 5 to 7 days, although residual testicular tenderness can persist for weeks. Testicular atrophy may follow orchitis in about 35% to 50% of cases, but sterility is an uncommon complication, even among patients with bilateral orchitis. Orchitis occurring in vaccinated males who develop “breakthrough” mumps tends to be less clinically severe (124).
Mumps can cause inflammation of other glandular tissues, including pancreatitis (125) and thyroiditis (126). Investigators in the 1970s proposed an epidemiologic association between mumps and juvenile diabetes mellitus (127); however, the dramatic decline in the incidence of mumps has not been reflected by a similar decline in the occurrence of juvenile-onset diabetes. Oophoritis and mastitis have been reported in postpubertal women with mumps (113,128). Renal function abnormalities are common in mumps and virus can be readily isolated from urine, but significant or permanent renal damage is rare (129,130). Other infrequent manifestations of mumps include arthritis (131,132), myocarditis (133,134), and thrombocytopenia (135).
Maternal mumps infection during the first trimester of pregnancy results in an increased frequency of spontaneous abortions (136). However, no clear association between congenital malformations and maternal mumps has been demonstrated (137). An etiologic relationship between gestational mumps and endocardial fibroelastosis has been postulated (138). Mumps occurring during the perinatal period is usually benign, perhaps due the partial protective effect of transplacental antibody (139).
Central Nervous System Manifestation of Mumps
CNS infection is the most common extrasalivary manifestation of mumps and may precede, accompany, or follow the development of parotitis (35). There is no association between the severity of the parotitis and the likelihood or severity of meningitis (27). Most frequently, CNS symptoms follow the onset of parotitis by about 5 days (140). In a study of 41 cases of mumps encephalitis reported by Koskiniemi et al. (31), parotitis appeared 3 to 14 days prior to CNS involvement in 15 patients, was coincident with encephalitis in 9 patients, occurred 1 to 4 days after the onset of CNS symptoms in 2 patients, and was absent in 15 patients. In another series of 24 patients with mumps parotitis and meningoencephalitis, 11 patients had parotitis for 6 to 21 days before the onset of the CNS symptoms, 9 patients had neurologic symptoms preceding the parotitis by 1 to 8 days, and 4 patients had parotitis and CNS symptoms that occurred simultaneously (42). Levitt et al. (30) noted a mean interval of 2.7 days between the onset of parotitis and the development of CNS symptoms, but the range was wide, with parotitis developing from 20 days before to 7 days after CNS disease. These data clearly indicate that the development of CNS disease in mumps does not depend on prior development of parotitis. The physician examining a patient with suspected meningoencephalitis may not exclude the possibility of mumps simply because the patient does not have clinically apparent salivary gland involvement.
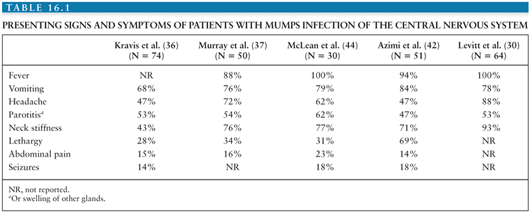
The common presenting signs and symptoms seen in patients with mumps CNS infection are summarized in Table 16.1. The most frequently reported presentation is a triad of fever, vomiting, and headache. Salivary gland enlargement is present in only 50% of patients with mumps CNS disease. The fever is frequently high (39°C to 40°C) and lasts for 72 to 96 hours. The headache and vomiting may also be quite severe and usually persist for about 48 hours (32,141). Other frequently noted clinical findings include neck stiffness, lethargy or somnolence, and abdominal pain. Most patients with mumps with CNS involvement have signs of meningitis (e.g., headache and nuchal rigidity), but no evidence of cortical dysfunction. Defervescence is usually accompanied by overall clinical recovery, and the total duration of illness in uncomplicated cases is 7 to 10 days (40,41).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree