TOXOPLASMA GONDII AND TOXOPLASMOSIS
JOSE G. MONTOYA
Toxoplasma gondii is an intracellular parasite of worldwide distribution infecting one third of the human population (1). The parasite has a notable tropism for the central nervous system (CNS) and maintains a highly clonal population structure in nature (2,3). Based on genotyping studies of mostly European and American strains, three main clonal lineages have been reported in animals and humans (types I, II, and III) (4–8). A fourth clonal lineage (type IV) has been recently proposed to circulate in the United States (9). In addition, atypical strains that do not fall in these four categories have also been reported as capable of causing disease in humans (10).
Type III strains are common in animals, whereas types I, II, or atypical strains are more commonly linked to human toxoplasmosis. Type II strains are more often associated with lower virulence in animal models of toxoplasmosis and less aggressive clinical manifestations in humans, whereas types I, III, and atypical strains are more often associated with worse outcomes in animal and human infections (11).
In Europe, mostly type II strains are observed, whereas in South America, type II strains are rarely found and atypical strains (in addition to type I and III) tend to predominate. In South America, infection with atypical strains has been associated with eye, pulmonary, and CNS infections in immunocompetent individuals (12,13). In North America, types II, I/III, IV, and atypical strains have been reported. In North America, atypical strains have also been associated with more severe ocular and congenital disease (10,14).
The infectious forms of T. gondii include the tachyzoite, the tissue cyst (containing bradyzoites), and the oocyst (containing sporozoites). The definitive host belongs to the Felidae family (e.g., cats); humans and other animals are only intermediate hosts (15). Humans can acquire the parasite by the oral route (e.g., ingesting food or water containing tissue cysts or oocysts), vertical transmission (i.e., tachyzoites from the acutely infected mother traversing the placenta), organ transplantation (i.e., tissue cysts contained in the allograft from an infected donor), or laboratory accident (e.g., in an experimental setting dealing with tachyzoites, tissue cysts, or oocysts) (16).
The tachyzoite is semilunar in shape, measures 2 to 3 µm wide and 5 to 7 µm long, and is responsible for the clinical manifestations observed in patients with toxoplasmosis during primary infection or reactivation (15,17). The tissue cyst measures up to 100 µm in diameter; it is responsible for the chronic infection (i.e., establishes latency in organs such as the brain, eye, heart, skeletal muscle, liver, and kidney) and for transmission via the ingestion of undercooked meat (17). Bradyzoites, crescent in shape and usually measuring 7 by 1.5 µm, can reactivate with the advent of significant immunosuppression and cause significant morbidity and mortality as they are transformed into tachyzoites (17). Oocysts are shed in soil and water by felids, measure 10 to 12 µm in diameter in the unsporulated form, and are responsible for the transmission to patients via inadvertent oral ingestion of infected cat feces; contaminated water, vegetables, or food; and contact with contaminated soil, for example, while gardening (17,18). Novel routes of transmission to humans have been recently described in the United States such as ingestion of raw shellfish (including oysters, mussels, and clams) (19). Transmission does not occur by direct contact with an infected person. Airborne transmission is unlikely (17).
EPIDEMIOLOGY
Acute infection can be asymptomatic and has been documented in individuals without history of conventional epidemiologic risk factors for acute toxoplasmosis (e.g., cat ownership or consumption of undercooked meat). Clinicians should not exclude the possibility of toxoplasmosis in patients who do not recall symptoms associated with acute infection or in patients who do not own cats or eat undercooked meat. Up to 50% of infected individuals do not have an epidemiologic history consistent with exposure to the parasite. Moreover, in addition to ingestion of undercooked meat containing tissue cysts, food or untreated water may be contaminated with oocysts (18).
Although primary infection is asymptomatic in most individuals, symptomatic patients with the acute infection can present with the following symptoms or syndromes, alone or in various combinations: fever, lymphadenopathy, headache, myalgias, arthralgias, sore throat, stiff neck, nausea, diarrhea, abdominal pain, anorexia, skin rash, confusion, earache, eye pain, general malaise, fatigue, chorioretinitis, hepatitis, myositis, or myocarditis. Disseminated disease, pneumonia, and even death have been observed in immunocompetent individuals infected with T. gondii in Latin America (12,13,20).
Following acute infection, a well-orchestrated immune response will transform the primary infection into a chronic (or latent) stage that lasts for the life of the host. Chronic infection with T. gondii in immunocompetent individuals has been traditionally viewed as asymptomatic. However, several investigators have suggested that chronic infection may be associated with a higher frequency of behavioral changes, traffic accidents, suicide, schizophrenia, or bipolar disorder (21,22). Significant T-cell–mediated immunodeficiency as observed in patients with AIDS, organ transplants, or those taking certain immunosuppressive drugs can result in clinically significant reactivation of the parasite in the CNS, eye, lungs, peripheral blood, heart, and skeletal muscle of chronically infected patients. The parasite can also cause significant disease in these patients when a seronegative transplant candidate acquires infection through the oral route or via the transplanted allograft (23).
Prevalence of chronic infection increases with age and varies highly between geographic locales and even within subpopulations of the same locale (24). The overall age-adjusted seroprevalence in the United States has been reported at 10%, but it may be as high as 30% in the Northeast region and higher among certain ethnic groups (25). In France, the estimated seroprevalence is 44%, whereas in South America and Africa, it can vary widely from 10% to 80% (26). It is important for each community to perform their own studies on the seroprevalence of the parasite because local climate, geographic factors, and hygienic and alimentary habits uniquely influence these rates.
The development of toxoplasmic encephalitis (TE) in immunocompromised patients depends on the prevalence of the infection in the general population, degree of immunosuppression, and genetics of host and parasite. Patients with hematopoietic stem cell, bone marrow, or cord blood transplants; with AIDS; or those receiving immunosuppressive drugs affecting cell-mediated immunity develop TE by reactivation of their latent infection. In contrast, solid organ transplants can develop TE through the transplanted organ when a seronegative recipient (R−) receives an organ from a seropositive donor (D+). However, seropositive (R+) solid organ recipients can also reactivate their latent infection under immunosuppression. Of note, in recent reports, patients with chronic lymphoproliferative disorders or taking immunosuppressive drugs affecting B-cell–mediated immunity have also been found at risk for TE by reactivation of their latent infection (27,28). The risk of developing TE by reactivation appears to be also influenced by the genetics of the host and parasite. Studies in mice suggesting that genetic factors in the host contribute to the development and severity of TE (29–31) and the fact that not all HIV-infected patients with positive T. gondii serologic findings develop TE suggested the possibility that genetic factors may also play a role in the vulnerability of immunosuppressed patients to develop CNS toxoplasmosis (32). The major histocompatibility complex (MHC) class II gene DQ3 (HLA-DQ3) has been significantly associated with the development of TE in North American Caucasian AIDS patients, whereas HLA-DQ1 was found to be marginally protective (32). HLA-DQ3 has also been significantly associated with the development of hydrocephalus in children with congenital toxoplasmosis (33). As noted earlier, parasite genetics appear to be an additional factor to predispose for TE because infection with atypical strains in South America has been associated with CNS infections in immunocompetent individuals (12,13).
Early studies indicated that 20% to 47% of T. gondii–seropositive AIDS patients ultimately developed TE in the absence of appropriate antitoxoplasma prophylaxis and highly active antiretroviral therapy (34). Following the introduction of effective antitoxoplasma prophylaxis and anti-HIV treatments, the risk of toxoplasmosis significantly decreased. The incidence in the United States of AIDS-associated TE declined from 2.1 per 100 person-years (PY) in 1992 to 0.7 per 100 PY in 1997 (35). However, TE remains as the most common cause of space-occupying brain lesions in HIV-infected patients whose CD4 counts are less than 200 cells/mm3 and are not taking effective antitoxoplasma prophylaxis and antiretroviral therapy (36). TE has been reported as an AIDS-presenting illness in patients who were not aware of their diagnosis of HIV infection (37).
Rarely, TE can occur among HIV-infected patients with CD4+ counts more than 200 cells/mm3 without brain-occupying lesions (i.e., diffuse encephalitis) or in seronegative patients. Brain biopsy or amplification of T. gondii DNA in cerebrospinal fluid (CSF) by the polymerase chain reaction (PCR) should be attempted in these patients in order to confirm the diagnosis of TE. If the diagnosis is confirmed among seronegative patients, they were likely recently infected by oral ingestion, reactivated their latent infection but were unable to produce detectable Toxoplasma antibodies, or were tested with serologic assays with low analytical sensitivity.
TE in immunocompetent individuals is rare but has been documented in the literature, and it is probably an underrecognized entity in these patients (38,39). In tropical areas of South America, life-threatening toxoplasmosis in immunocompetent patients has been associated with atypical strains of the parasite (13,40). TE should be included in the differential diagnosis of travelers returning from these areas and presenting with encephalitis of unknown etiology.
The CNS is one of the prominent sites of infection in infants congenitally infected with T. gondii. The parasite can cause severe TE (including hydrocephalus, brain parenchymal lesions, and calcifications) and chorioretinitis in infected infants. Congenital TE is rarely observed in countries (e.g., France) where infants are born to mothers who are screened and receive treatment for toxoplasmosis during gestation (41). In contrast, TE is relatively frequent in infected infants born to mothers who do not receive this benefit. In a recent study from the United States, severe TE was diagnosed in congenitally infected infants whose mothers had not received antitoxoplasma treatment during gestation. Brain calcifications were seen in 94 (79.6%) of 118 and hydrocephalus was present in 67 (67.7%) of 99 infected infants who have been referred to a national reference laboratory for laboratory confirmation of their diagnosis of congenital toxoplasmosis (42).
CLINICAL MANIFESTATIONS
Reactivation in Immunosuppressed Patients
Toxoplasmosis should be suspected in any immunocompromised patient with encephalitis (with or without focal brain lesions), seizures, pneumonia, chorioretinitis, fever of unknown origin, myocarditis, polymyositis, or hepatitis because several major hospitals have encountered patients who died of disseminated toxoplasmosis that was only diagnosed at autopsy (43–53). The CNS is often recognized as the only and most common site for reactivation of toxoplasmosis in immunocompromised patients. However, other organs can be concomitantly involved, including lungs, eye, heart, and adrenal glands. Moreover, reactivation in these patients can also occur in extraneural sites in the absence of TE or manifested only as fever of unknown origin. Multiple areas of the brain are usually affected, but there is a preference of the parasite for the gray matter on histologic examination and for the basal ganglia in brain imaging studies. TE has a wide spectrum of clinical manifestations, including alteration of mental status, seizures, motor weakness, sensory abnormalities, cerebellar dysfunction, meningismus, movement disorders, and neuropsychiatric manifestations. The typical presentation is subacute onset of focal neurologic abnormalities seen in 58% to 89% of patients (54–57). It should be noted that patients with or without brain space-occupying lesions may have nonfocal exams. In fact, altered mental status, manifested by confusion, lethargy, delusional behavior, frank psychosis, global cognitive impairment, anomia, or coma, may be present initially in as many as 60% of patients (54,56,58). Seizures are the reason for seeking medical attention in approximately one third of AIDS patients with TE (54,56,58,59). Focal neurologic deficits are evident on neurologic examination in approximately 60% of patients (54,56,58,59). Hemiparesis is the most common focal neurologic finding. Additional focal findings include visual field loss, cranial nerve palsies, aphasia, ataxia, dysmetria, hemichorea-hemiballismus, tremor, parkinsonism, akathisia, or focal dystonia. In addition, involvement of the spinal cord with T. gondii has been described with cases of transverse myelitis and conus medullaris syndrome (60). Ventriculitis accompanied by hydrocephalus may also be seen (61).
A diffuse form of encephalitis, without space-occupying lesions in imaging studies, usually with a rapidly fatal clinical course, has been reported (62,63). Although rare, T. gondii should always be entertained as a possible etiologic agent in any patient with unexplained encephalitis or meningoencephalitis, with or without space-occupying lesions in brain (64).
Extracerebral sites of Toxoplasma reactivation in immunocompromised patients can cause significant morbidity and mortality and present with (40%) or without (60%) concomitant TE (65). TE must be ruled out by brain imaging studies even in the absence of obvious CNS symptoms in any immunocompromised patient with extracerebral toxoplasmosis. The prevalence of extracerebral toxoplasmosis in AIDS patients has been estimated at 1.5% to 2.0% and usually occurs when their CD4 counts of less than 100 cells/mm3 (mean: 57 cells/mm3) (65). Among patients with extracerebral toxoplasmosis, the sites of involvement include the eyes (50% of patients); lung (26%); disseminated (at least two extracerebral visceral sites) (11.5%); peripheral blood (acute febrile syndrome with isolated positive parasitemia) (3%); heart (3%); bone marrow (3%); bladder (1%); and isolated cases of rhinopharynx, skin, liver, lymph nodes, conus medullaris, and pericardium (65). In addition, skeletal muscle and pancreatic involvement were always associated with other extracerebral toxoplasmosis sites (65). Toxoplasmosis can occasionally present as septic shock in patients with TE, with or without involvement of extracerebral sites (66). Toxoplasmic pneumonitis is greatly underdiagnosed because clinicians do not readily consider T. gondii among the etiologic agents of pneumonia. The most common clinical syndrome of toxoplasmic pneumonia is prolonged febrile illness with cough, hypoxemia, and dyspnea that is clinically indistinguishable from Pneumocystis jiroveci pneumonia (PCP) infection. Like PCP, toxoplasmic pneumonia most often results in bilateral ground glass opacities on chest computed tomography (CT) (no contrast is required) and can result in acute respiratory distress syndrome (67).
Toxoplasma reactivation in immunosuppressed patients can also manifest as fever and sepsis-like syndrome with hypotension, disseminated intravascular coagulation, elevated lactate dehydrogenase, and pulmonary infiltrates. This syndrome may not be associated with clinical or radiologic evidence of TE (66).
Toxoplasmic chorioretinitis occurs relatively infrequently in AIDS patients (when compared with the incidence of cytomegalovirus retinitis) (68). Floaters and loss of visual acuity are common complaints and may be accompanied by ocular pain. In AIDS patients, funduscopic examination typically reveals findings consistent with necrotizing retinochoroiditis. The lesions are yellow-white areas of retinitis with fluffy borders; ocular lesions are usually located away from areas of preexisting scars. Vitreal inflammation may vary from mild localized vitreal haze to severe vitreous inflammation. Vasculitis and hemorrhage are uncommon. These findings suggest that the pathogenesis of these lesions may be secondary to hematogenous seeding rather than local reactivation of previous foci of infection in the retina. The presence of concurrent TE in AIDS patients with ocular toxoplasmosis has varied from 29% to 63% (68). Features of toxoplasmic retinochoroiditis commonly observed in the immunocompetent host (e.g., areas of inflammation stemming from brown-greenish scars with a light in the fog appearance) may be absent in patients with AIDS.
Primary Infection in Immunocompetent Patients
Symptoms and signs suggestive of encephalitis, meningoencephalitis, or focal brain lesions have been reported in immunocompetent individuals with TE (13,38–40). Severe headache, neck pain, and meningismus are common complaints.
Congenital Disease
Vertical transmission can occur in pregnant women who acquire primary Toxoplasma infection during gestation or in severely immunosuppressed women who reactivate chronic Toxoplasma infection during gestation (e.g., pregnant women with AIDS who develop TE). It has also been described in women who acquire the infection shortly before conception (e.g., within 3 months of initiation of pregnancy).
The CNS is one of the main sites of infection in congenital toxoplasmosis. Clinical manifestations of CNS involvement by T. gondii in the fetus include hydrocephalus and brain calcifications. Clinical manifestations in the infant include chorioretinitis, strabismus, blindness, seizures, encephalitis, abnormal cephalic perimeter (microcephaly or hydrocephalus), and psychomotor or mental retardation. Intracranial calcifications or hydrocephalus are visible on brain imaging studies. Children can continue suffering the chronic sequelae of the congenital disease. However, children and adults may become symptomatic for the first time during this phase of their lives, primarily in the form of reactivation of the congenitally acquired chorioretinitis.
DIAGNOSIS
At present, the definitive diagnosis of TE can be accomplished by (a) amplification of T. gondii DNA from CSF (e.g., PCR), (b) visualization of the tachyzoite form in CSF or CNS tissue (e.g., Wright-Giemsa or hematoxylin-eosin stains), (c) visualization of tissue cysts surrounded by inflammatory cells in CNS tissue, or (d) isolation of the parasite from CSF. Toxoplasma IgG and IgM testing in CSF is not useful and should be abandoned as a diagnostic aid. If CSF is available in limited amount (as it is usually the case), PCR is the preferred diagnostic method. Other diagnostic aids used toward supporting the diagnosis of TE include detection of Toxoplasma-specific immunoglobulins (typically immunoglobulin [Ig] G and IgM) in serum, brain imaging studies, response to Toxoplasma-specific empiric treatment (e.g., pyrimethamine/sulfadiazine/folinic acid), and isolation of the parasite from body fluids. In most cases of TE, CSF general examination is normal or may reveal mild pleocytosis (predominantly lymphocytes and monocytes) and an elevated protein level, whereas the glucose content usually is normal.
Polymerase Chain Reaction
Toxoplasma PCR testing in CSF for the detection of parasite DNA is highly specific (69,70). A positive test result in CSF (without concerns of laboratory contamination) establishes the diagnosis of TE in any clinical setting. This is not the case for PCR of brain/spinal cord tissue because T. gondii cysts can persist in these tissues for the life of the host and a positive test result may be indicative of latent infection rather than toxoplasmosis by reactivation. PCR can also be helpful to support the diagnosis of TE when other body fluids are used in immunosuppressed patients (e.g., peripheral blood, bronchoalveolar lavage, vitreous fluid, aqueous humor, and possibly urine). In addition to TE, examination of the CSF by PCR can also aid in the diagnosis of JC virus (JCV)–associated progressive multifocal leukoencephalopathy (PML), Epstein-Barr virus (EBV)–associated CNS lymphoma, and cytomegalovirus (CMV) ventriculitis.
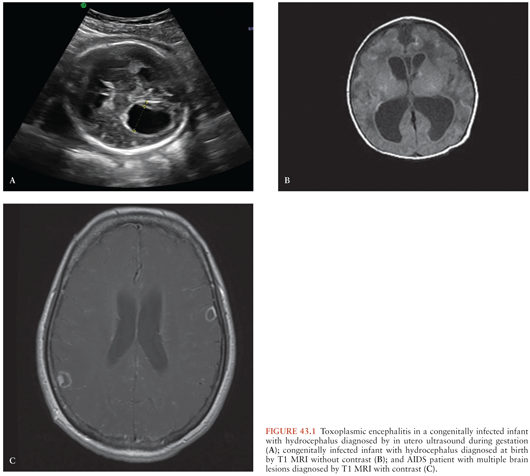
During gestation, a positive PCR test result in amniotic fluid is diagnostic of congenital TE in a fetus whose imaging studies (fetal ultrasound or MRI) have revealed hydrocephalus or brain calcifications (Fig. 43.1). Of note, attempts to perform prenatal diagnosis of congenital infection using PCR in amniotic fluid of pregnant women dually infected with HIV and T. gondii had been hampered by the fact that amniocentesis was contraindicated in HIV-infected mothers because of the potential of transmission of HIV to the fetus during the procedure (71). However, in a recent study by Mandelbrot et al. (72), the risk of maternal to child transmission of HIV by amniocentesis was negligible in mothers taking antiretroviral therapy (ART). Thus, in HIV-positive women who acquired their primary T. gondii infection during gestation or in women with AIDS who develop TE, amniocentesis to investigate whether fetal infection has occurred is indicated assuming that these women are taking their ART and their HIV viral load is undetectable (72).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree