HUMAN IMMUNODEFICIENCY VIRUS
CHRISTINA M. MARRA
HIV can directly infect the cerebrospinal fluid (CSF) and the brain. Infection of the CSF is a notable feature of the acute retroviral syndrome, and CSF pleocytosis is common in HIV infection, particularly among individuals with high peripheral blood CD4+ T-cell counts, detectable plasma HIV RNA, and in those not on antiretroviral therapy (1). Infection of the brain likely is the substrate for dementia in HIV infection. The immunodeficiency caused by HIV predisposes individuals to secondary or opportunistic central nervous system (CNS) infections or neoplasms (Table 19.1). The incidence of dementia and most CNS opportunistic infections has declined in the developed world with widespread use of combination antiretroviral therapy (CART). However, although the incidence may be decreasing, the prevalence of cognitive impairment is increasing (2–4). In addition, treatment of HIV itself may be associated with neurologic complications. The clinical findings and treatment of CNS opportunistic infections seen in HIV-infected individuals are covered in several chapters of this book. In particular, a detailed discussion of cerebral toxoplasmosis is provided in Chapter 43, and neurosyphilis is covered in Chapter 38. This chapter addresses two opportunistic infections not covered elsewhere: primary CNS lymphoma, which is considered with infections because of its association with Epstein-Barr virus infection, and progressive multifocal leukoencephalopathy (PML) caused by the JC virus. In addition, the most common CNS complications associated with HIV itself are discussed, including newly recognized syndromes.
FOCAL BRAIN DISEASE
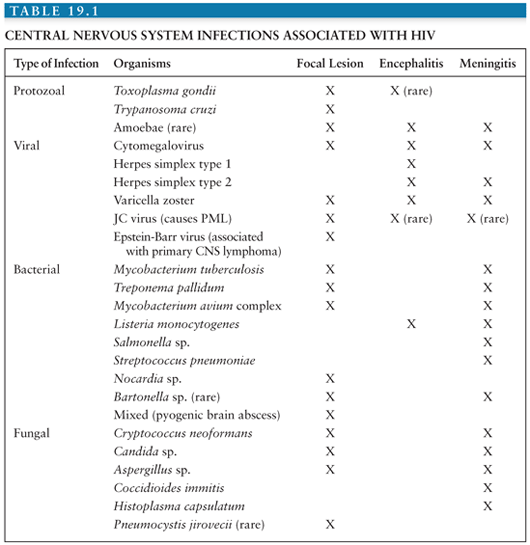
Opportunistic infections, neoplasms, and cerebrovascular disease are the most common causes of focal findings referable to the brain in HIV-infected patients. In such individuals, the presence or absence of contrast enhancement and mass effect on cranial neuroimaging studies helps to narrow the differential diagnosis. Lesions associated with contrast enhancement and mass effect are most commonly due to toxoplasmosis, primary CNS lymphoma, or tuberculoma. Lesions with minimal or no contrast enhancement and no mass effect are most commonly due to PML.
Primary Central Nervous System Lymphoma
Etiology
Primary CNS lymphomas in HIV-infected patients are typically of B-cell origin and are classified as diffuse large cell or immunoblastic (5). Epstein-Barr virus (EBV) is detectable in these tumors in virtually all HIV-infected patients (5). Prolonged immune suppression and EBV-induced B-cell stimulation likely contribute to tumor development (6).
Differential Diagnosis
As noted earlier, the most common causes of brain lesions associated with contrast enhancement and mass effect are toxoplasmosis, primary CNS lymphoma, and tuberculoma. Other causes are listed in Table 19.1.
Clinical Symptoms and Findings
The presenting clinical features of HIV-associated primary CNS lymphoma include confusion, lethargy, memory loss, hemiparesis, speech and language disorders, seizures, and cranial nerve palsies (7,8).
Laboratory and Imaging Studies
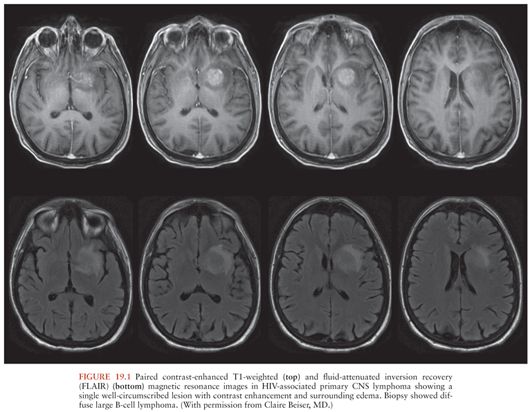
HIV-infected patients with primary CNS lymphoma typically have peripheral blood CD4+ T-cell counts less than 50/µL (9). Brain computed tomography (CT) or magnetic resonance imaging (MRI) show ring- or homogeneously enhancing lesion(s), often located periventricularly or in the frontal lobes, although other locations may be seen (Fig. 19.1). These lesions may cross the midline in the corpus callosum and may be associated with patchy nodular ventricular enhancement (10). Thallium-201 (201Tl) single-photon emission computed tomography (SPECT) may be helpful in distinguishing between CNS infections and primary CNS lymphoma in HIV-infected individuals. Focal areas of increased 201Tl uptake are seen in patients with lymphoma, whereas no brain uptake is seen in patients with CNS infections such as Toxoplasma encephalitis or tuberculoma (11,12). Delayed imaging and calculation of a retention index increase the specificity of the test (12).
Although the diagnosis of primary CNS lymphoma can only be proven by histopathology, examination of CSF for EBV DNA by polymerase chain reaction (PCR) may be sensitive and specific for establishing the diagnosis (13) when it is used in the appropriate clinical setting: an HIV-infected patient with a focal brain lesion with mass effect and enhancement. The positive predictive value of the test is highest in patients with a low likelihood of CNS toxoplasmosis, such as those who are Toxoplasma seronegative or who have been taking trimethoprim-sulfamethoxazole for prophylaxis against toxoplasmosis. When the test is used more broadly in clinical settings, the positive predictive value of detection of EBV DNA in CSF is lower (14,15).
Treatment and Prevention
HIV-associated primary CNS lymphomas are sensitive to radiation therapy. In a retrospective analysis conducted before the availability of CART, median survival was about 3 to 4 months with radiation therapy and dexamethasone and untreated survival was 3 to 4 weeks (16). Death was usually due to opportunistic infection rather than lymphoma (7,8). Absence of opportunistic infection, younger age, higher Karnofsky performance status, and delivery of higher biologically effective doses of radiation were associated with longer survival (17).
More modern studies show that whole brain radiation therapy (at least 30 Gy) (18–20) and CART (18,20,21) are associated with better survival in HIV-infected patients with primary CNS lymphoma, often with best survival seen in those who receive both modalities. Prolonged survival has also been reported in HIV-infected patients with primary CNS lymphoma who only received CART (22–24). Some have suggested that whole brain radiation be reserved for patients who do not respond to antiretrovirals to avoid the morbidity, particularly leukoencephalopathy, associated with this treatment modality. Because of the association with EBV, some investigators have advocated combination therapy for HIV-associated primary CNS lymphoma that targets this virus with agents such as ganciclovir or foscarnet. Individual reports of success with these regimens can be difficult to evaluate because patients are often also treated with radiation, CART, and immune modulators (25–30). Overall, treatment data for HIV-associated primary CNS lymphoma are mostly from retrospective case reports and series and are limited by selection bias. The best treatment for HIV-associated primary CNS lymphoma is not known because a randomized trial has not been conducted, and, given the low incidence of the disorder, may never be feasible.
Prior to the advent of CART, primary CNS lymphoma occurred in 1% to 4% of HIV-infected persons. Since CART has become widely available, the incidence of this disorder has declined (31,32) and survival has improved (18,33). Thus, prevention lies in antiretroviral treatment to prevent prolonged immunosuppression.
Progressive Multifocal Leukoencephalopathy
Etiology
PML is caused by a polyoma virus called JC virus. This virus is usually acquired in childhood. It remains latent in kidney and perhaps in brain, reactivates in the setting of immunosuppression, and infects oligodendrocytes and less so astrocytes. Death of oligodendrocytes leads to demyelination.
Differential Diagnosis
The diagnosis of PML should be considered in patients with dementia, particularly if there are also focal neurologic findings. Varicella-zoster encephalitis may cause demyelination and should also be considered (34). Cytomegalovirus (CMV) encephalitis is sometimes accompanied by focal lesions that could mimic PML (35). Substance abuse, particularly a form of heroin use called “chasing the dragon,” can cause clinical and radiographic abnormalities similar to PML (36). A severe form of HIV-associated leukoencephalopathy characterized pathologically by extensive perivascular macrophage infiltration and demyelination and high levels of brain and CSF HIV RNA has been described (37). Patchy or confluent white matter high signal intensity is seen on brain MRI. The syndrome occurs in patients failing CART and is accompanied by cognitive abnormalities. Leukoencephalopathy has been described in individuals whose HIV is successfully treated, and has been attributed both to “CNS immune reconstitution” (38–40) and to “CNS escape” in individuals with suppressed plasma HIV RNA but detectable CSF HIV RNA (41). The latter two diagnoses may be distinguished from PML because they may be associated with CSF pleocytosis.
Clinical Findings
Patients with PML typically experience insidious onset of progressive neurologic deficits, most commonly cognitive dysfunction, limb weakness, gait disturbance, coordination difficulties, and visual loss. Headache is a complaint in about one fourth of patients. Neurologic examination shows focal deficits, particularly hemiparesis or visual field abnormalities (42,43). Less common manifestations of CNS JC infection include cerebellar granule cell neuronopathy (44) and JC infection of cortical pyramidal neurons, causing an acute encephalopathy (45).
Laboratory and Imaging Studies
HIV-infected patients with PML typically have peripheral blood CD4+ T cells less than 200/µL, although even before the advent of CART, about 10% of patients had CD4 counts above this threshold (43). In the current treatment era, individuals with PML and much higher or even near-normal peripheral blood CD4+ T cell concentrations have been reported (46,47).
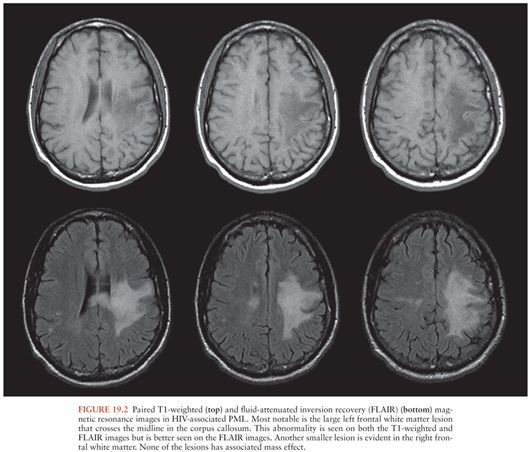
Brain CT in patients with PML may sometimes be normal. More often, it shows multiple, often confluent, white matter lesions that are most commonly located in the parietooccipital regions. These lesions are low density and have little, if any, mass effect (43). Enhancement may be seen in 10% of CT scans and is usually faint and peripheral (43). Brain MRI shows more lesions than CT (48). In contrast to the white matter lesions seen in HIV-associated dementia, which are visible only on T2-weighted images, PML lesions are low intensity on T1-weighted and high intensity on T2-weighted images (Fig. 19.2); about 15% show faint contrast enhancement (43). Contrast enhancement is more likely to be seen in the setting of immune reconstitution (see the following discussion). In addition to the abnormalities seen on T1- and T2-weighted MRI, restricted diffusion may be seen; additional examples of MRI abnormalities in PML are shown in Chapter 3.
Diagnostic criteria for PML have been recently published (49). In patients with characteristic clinical and neuroimaging findings, histologic examination or identification of JC virus DNA by PCR in CSF can confirm the diagnosis. However, a negative PCR does not exclude the diagnosis. Individuals who develop PML while receiving CART as well as those with higher peripheral blood CD4+ T cell concentrations are more likely to have a negative diagnostic PCR (50).
Treatment and Prevention
Recent studies suggest that the current incidence of PML has declined approximately three- to fourfold compared to before 1996, with estimates among HIV-infected individuals ranging from 0.6 to 1.3 per 1,000 person-years (51,52). Treatment with potent antiretrovirals has also resulted in significantly improved survival in patients with PML (51–53).
Several prognostic factors have been identified in HIV-associated PML. Mass effect on MRI (54) and brainstem and cerebellar involvement (55,56) are associated with poorer outcome. Higher peripheral blood CD4+ T-cell count at diagnosis (48,51,57–60), PML as the AIDS-defining illness (58), lower concentration and decline in CSF JC virus DNA concentration during antiretroviral therapy (59–64), and the presence of JC virus-specific cytotoxic T cells in blood (65–67) are associated with improved survival.
All HIV-infected patients with PML should be treated with CART aimed at complete suppression of plasma HIV viremia. Whether certain types of antiretroviral regimens are more effective than others remains a matter of debate because few prospective studies have been conducted. Two retrospective analyses suggest that a regimen that includes a protease inhibitor may be more effective than one that does not (68,69). Gasnault and colleagues (60) conducted a multicenter, prospective, open-label trial of an individualized regimen of five antiretrovirals, including enfuvirtide for the first 6 months, in 28 HIV-infected patients with PML. The 1-year survival was 75%, significantly higher than the historically reported survival of 45% at the time the trial was planned (63,70–72). In this trial, all deaths occurred within the first 4 months of treatment (60).
As a consequence of an immune reconstitution inflammatory syndrome (IRIS), PML may develop (unmasking or simultaneous IRIS) or worsen (paradoxical or delayed IRIS) after beginning antiretroviral therapy. In an observational study in Spain of 61 patients with PML, 14 (23%) developed PML IRIS, of which 8 were unmasking IRIS and 6 paradoxical IRIS (53). In a case series and retrospective review, PML IRIS developed 1 week to 26 months (median 7 months) after beginning antiretroviral therapy, with a shorter latency, greater number of MRI-defined brain lesions, and poorer outcome in paradoxical compared to unmasking IRIS (73). A retrospective case series showed that peripheral blood CD4+ T cells & <50/µL at the time of initiating antiretroviral therapy significantly increases the risk of PML IRIS and that the prognosis of patients with PML IRIS is no different than PML patients without IRIS (74).
Paradoxical IRIS is characterized by clinical worsening and progression of previously defined MRI lesions or development of new lesions, often, but not always, with evidence of contrast enhancement. In the absence of new enhancement, paradoxical IRIS can be difficult to distinguish from progressive disease, although the onset of clinical worsening in IRIS may be more acute. Both fatal and benign courses have been described (73,75–77) as well as individuals that do and do not respond to immunosuppression with steroids (73,78). A single case of rapid improvement in paradoxical PML IRIS after treatment with the CCR5 inhibitor, maraviroc, but not treatment with methylprednisolone, has been reported (79). The authors speculated that blocking of CCR5+ leukocyte recruitment to the CNS might have been the underlying mechanism of improvement.
No specific therapy for HIV-associated (or nonassociated) PML has been identified. Cytosine arabinoside (Ara-C) given intravenously (IV) or intrathecally did not confer a survival benefit in a clinical trial conducted before the availability of CART (80). A metaanalysis of cidofovir showed no benefit beyond that of CART (72). Mefloquine showed anti-JC activity in an in vitro assay (81), but an open-label randomized trial was stopped early because of lack of efficacy (64). Based on the observation that the cellular receptor for JC virus is the 5-HT2A serotonin receptor (82), mirtazapine has been used for PML treatment with anecdotal reports of success (83,84), but no clinical trial has been conducted.
HIV-infected patients with PML who survive generally have persistent neurologic deficits, although some regain independence. In the trial by Gasnault and colleagues referenced earlier, the median modified Rankin scale at 12 months in the 21 survivors was 3 (moderate disability). Eight patients had only slight disability and were independent (60). In contrast, in the Swiss HIV Cohort Study, among 47 individuals who survived more than 1 year, only 8 (17%) had clinical improvement (52). In a convenience sample of 23 HIV-infected patients with PML who survived at least 5 years, 9 remained neurologically stable, 10 had partial improvement, and 4 had marked improvement; 8 had only slight disability and lived independently (55). In a population-based study from Denmark, 5 of 11 patients followed for 3 years (from an original group of 47 patients) returned to their pre-PML level of function (51).
DIFFUSE CENTRAL NERVOUS SYSTEM DISEASE
HIV-Associated Neurocognitive Disorders
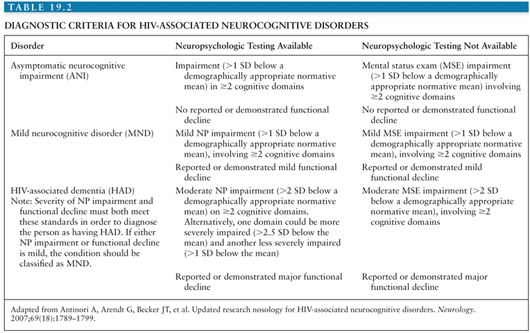
Cognitive impairment due to HIV infection was first characterized by Navia et al. (85) in 1986 and termed AIDS dementia complex or ADC to emphasize a triad of cognitive, motor, and behavioral abnormalities: (a) forgetfulness and loss of concentration; (b) apathy, social withdrawal, and irritability; and (c) loss of balance and leg weakness. On neurologic examination, such patients typically demonstrated slowed verbal and motor responses, reminiscent of neurologic findings in Parkinson disease. Not every patient with ADC had all three components of the triad at onset of disease, and not all patients with mild disease progressed to more severe disease. As will be discussed further in the following section, with the advent of CART, the incidence of HIV-associated dementia (HAD) has decreased, but the incidence and prevalence of less severe forms of cognitive impairment have increased. In 2006, a working group addressed the criteria for diagnosis of HIV-associated neurocognitive disorders (HAND) (86). These criteria are commonly referred to as the “Frascati criteria,” because the working group met in Frascati, Italy. Three categories of impairment were established: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HAD. The diagnosis of all three disorders is based on results of neuropsychologic (NP) tests covering at least the domains of verbal/language, attention/working memory, abstraction/executive, memory (learning, recall), speed of information processing, and sensory–perceptual or motor skills, in combination with an assessment of everyday functioning. In all categories, cognitive or functional impairment cannot be explained by delirium, opportunistic CNS disease, systemic illness, psychiatric illness, substance use, or medications with CNS effects (Table 19.2). Although these categories were intended for research purposes, they have become increasingly applied to clinical settings.
Epidemiology and Risks
Estimates early in the HIV epidemic suggested that at least 60% of patients with AIDS developed overt or subclinical HAD (85,87). The proportion decreased to about 7% in the first years after antiretrovirals became available (88,89). Several studies in the CART era show that the prevalence of HAD is low but the prevalence of milder impairment (MND and ANI) is surprisingly high. For example, in the multicenter U.S. CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study, among 1,316 participants without severe confounding disorders that could independently explain cognitive impairment, 2% had dementia, 12% had MND, and 33% had ANI (90). In a study of 100 Italian patients with undetectable plasma HIV RNA, 2% met criteria for HAD, 17% for MND, and 50% for ANI (91), and in a study of 400 French patients, 7% had HAD, 31% had MND, and 21% had ANI (92). Although symptomatic HAND (MND and HAD) remains a risk factor for poorer survival (4), the relevance of an ANI diagnosis has been questioned for several reasons. These include concerns that (a) normative NP data are from nonconfounded “normal” individuals rather than background-matched controls; (b) in a normally distributed population, 16% will score 1 SD below the norm on a given test; and (c) no published study has demonstrated that ANI confers a risk for subsequent dementia (93,94). In addition, patients frequently transition from mild impairment to normal cognition and vice versa despite stable CART regimens (95,96). Whether this reflects diagnostic imprecision, practice effects or normal variation remains undefined (97).
Low current, and in particular low nadir, CD4 is a risk factor for prevalent neurocognitive impairment in the CART era (90,95,98,99). However, the association between HIV-related factors and incident cognitive impairment is less clear. Although current but not nadir CD4 remained a significant predictor of incident dementia in the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) cohort (100), in the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT) study, neither current nor nadir CD4 or plasma HIV RNA predicted incident cognitive impairment (95). In a population-based study of 1,320 HIV-infected patients in Canada, incident symptomatic cognitive impairment was more common with lower nadir CD4 and plasma HIV RNA greater than 1 million copies/mL (101). In this study and in the CASCADE study, longer estimated duration of HIV infection conferred greater risk of incident impairment (100,101).
Etiology
HIV can be recovered from CSF (102) or brain (103) early in the course of infection, and acute meningitis or encephalitis may be part of the acute retroviral syndrome (102,104,105). Moreover, brain inflammation and injury is evident in primary HIV infection in some, but not all, patients (106–108). Most experts agree that productive brain HIV infection is restricted to perivascular macrophages and less so microglia (109). Infection of the CNS may be driven by activation of peripheral monocytes as a consequence of microbial translocation from the gut early in infection (110). Activated monocytes are better able to support productive HIV infection (111), and HIV DNA concentration in activated monocytes is higher in patients with cognitive impairment than in those with normal cognition (112). Astrocytes may be infected but do not support viral replication (113). The prevailing view is that HIV causes brain injury and subsequent cognitive impairment via indirect mechanisms (114–116). Proposed models suggest that HIV-infected mononuclear phagocytes release toxic viral gene products such as gp120 or tat. Alternatively, infected brain macrophages or microglia may release cell-derived toxins such as quinolinic acid; cytokines, including tumor necrosis factor-α (TNF-α); eicosanoids; platelet-activating factor; or nitric oxide. This hypothesis is particularly compelling because the neuropathology of HAD is characterized by increased numbers of activated macrophages, and the severity of dementia correlates better with the degree of macrophage staining in brain than with the number of HIV-infected cells in brain (117). Toxic substances released by activated macrophages may injure neurons directly, may injure astrocytes or oligodendrocytes and interfere with their supporting functions, or may stimulate astrocytes or oligodendrocytes to release toxic products that may augment neurotoxicity. Nonproductive HIV infection of astrocytes may contribute to macrophage activation, a 180 augmenting neurotoxicity.
The relationship between brain HIV infection and clinically evident cognitive impairment may be changing in the current treatment era, particularly among patients with mild impairment. A recent pathologic study of 589 individuals with advanced disease showed no relationship between brain HIV infection and HAND (118), and, as discussed in the following section, although detectable CSF HIV was associated with prevalent dementia and predicted it in the pre-CART era, these associations are less robust in the era of CART. Moreover, the relationship between lower nadir CD4 and cognitive impairment suggests that CNS injury may have occurred in the past, and that although impairment may be persistent, it may not reflect the effect of ongoing CNS infection, inflammation, or injury. This “legacy,” however, might lessen cognitive reserve. As HIV-infected patients live longer, aging (119), vascular disease (120–122), metabolic abnormalities (123), and CART toxicities (124–126) may increasingly contribute to cognitive impairment.
Differential Diagnosis
Before the advent of highly active antiretroviral therapy, HAD was a disease of those with advanced immunosuppression, and CMV encephalitis was an important alternative diagnosis to consider. In the current treatment era, patients with HAD may present with peripheral blood CD4+ T cells more than 200 cells/µL (2,3) and CMV encephalitis is rare. The differential diagnosis includes psychiatric disease, particularly depression; adverse effects from prescription or illicit drugs; and cerebral opportunistic infections including toxoplasmosis, tuberculosis, cryptococcal meningitis, and neurosyphilis. Rarely, PML may present solely with cognitive changes, but focal neurologic findings and neuroimaging almost always distinguish patients with PML from those with HAD (127).
Clinical Symptoms and Findings
Patients with HAD generally present with subacute onset of cognitive impairment, often with complaints of mental and physical slowness. Neurologic examination typically is remarkable for slowed verbal and motor responses. Ataxia and hyperreflexia may also be seen. A staging system for HAD has been used to characterize clinical severity (128,129). It is based on functional disability and ranges from normal cognitive and motor function (stage 0), to mild impairment (stage 1) with preserved ability to perform all but the more demanding tasks of work or daily activities, to end-stage disease (stage 4) distinguished by rudimentary comprehension and responses. Not all patients with mild disease progress to more severe stages.
Laboratory and Imaging Studies
The diagnosis of HAD is based on clinical findings and remains one of exclusion. There is no specific test that establishes the diagnosis. However, CSF studies may be the most informative. Conventional CSF evaluation helps exclude other disorders. Data from the Multicenter AIDS Cohort Study suggest that a CSF β2-microglobulin concentration more than 3.8 mg/dL in a CSF specimen with a normal white blood cell (WBC) count is specific, but not sensitive, for the diagnosis of HAD (130). In the pre-CART era, among patients with AIDS, CSF HIV RNA levels were elevated in those with cognitive impairment (131); they correlated with severity of dementia (132,133) and predicted incident impairment (134). In the CART era, CSF HIV RNA does not distinguish between those with and without cognitive impairment (135,136). However, in the clinical setting, measurement of CSF HIV RNA can be useful. A CSF concentration higher than plasma suggests, but does not prove, that cognitive impairment is due to ongoing CNS HIV infection and is one definition of CNS escape (see the following discussions).
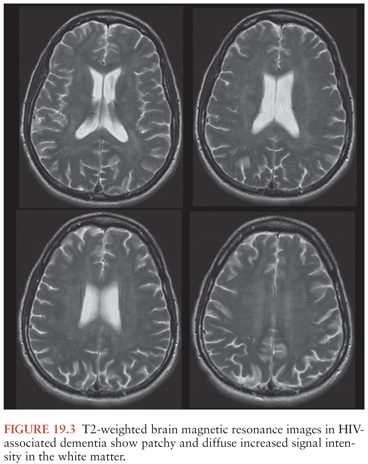
Neuroimaging is useful in excluding other disorders but does not establish the diagnosis of HAD. Cranial CT may be normal or show atrophy or patchy white matter attenuation (85). Cranial MRI is more sensitive than CT for demonstrating white matter abnormalities and may show high T2 signal in the periventricular regions and in the centrum semiovale that are not seen on T1-weighted sequences (Fig. 19.3). However, atrophy and focal white matter abnormalities may be seen in HIV-infected individuals without cognitive changes (137).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree