TRYPANOSOMIASIS
LOUIS V. KIRCHHOFF
Trypanosomes are single-celled protozoan parasites that have an amazingly broad distribution in nature. The diversity and wide geographic range of the animals infected by trypanosomes make one wonder if they do not impart a survival advantage to the species that harbor them. Trypanosomes are found in both warm- and cold-blooded vertebrates and also in a large number of invertebrates that often act as vectors. Simply stated, if an animal has blood or a bloodlike substance, there is a good chance that it is a host for trypanosomes. Trypanosomes were first observed in 1841 by Gabriel Valentin, who saw the motile organisms while examining blood from a trout under a microscope. Two years later, David Gruby saw trypanosomes in the blood of a toad and noted their undulating membranes. They were first seen in mammalian blood by Timothy Lewis, who observed them while studying rats. Infection with trypanosomes in human blood was noted for the first time in an English boat captain traveling on the Gambia River in the early years of the twentieth century. That species was later named Trypanosoma gambiense (1). Shortly thereafter, the Brazilian physician Carlos Chagas described the cycling of trypanosomes between the insect (triatomine or kissing bug) vectors and wild mammals and ultimately found the organisms in an ill child. He named the parasite Trypanosoma cruzi, after his mentor Oswaldo Cruz, and appropriately, the illness became known as Chagas disease or American trypanosomiasis (2). Although there are many members of the genus Trypanosoma, only T. cruzi and two African trypanosome subspecies, Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense, are capable of causing disease in humans. There are only two situations in which T. cruzi causes disease of the central nervous system (CNS). The first is the uncommon occurrence of meningoencephalitis during the acute phase of Chagas disease, which occurs most often in children. The second is cerebral reactivation of chronic T. cruzi infection in immunosuppressed patients, particularly in persons infected with the human immunodeficiency virus (HIV). In contrast, in patients with human African trypanosomiasis (HAT), caused by T. brucei rhodesiense and T. brucei gambiense, CNS disease is the most important clinical problem.
AMERICAN TRYPANOSOMIASIS
Life Cycle and Mechanisms of Transmission
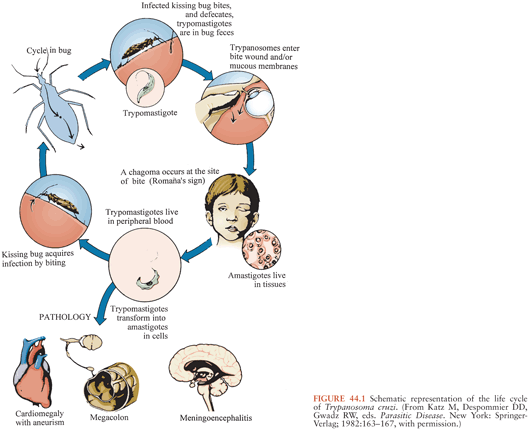
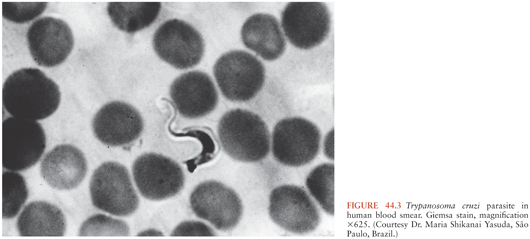
T. cruzi has a complex life cycle that involves insect vectors and mammalian hosts (Fig. 44.1). The vectors, often called kissing bugs or triatomines (3) (Fig. 44.2), become infected when they take a blood meal from mammals that have parasites circulating in their blood (Fig. 44.3). The organisms then multiply extracellularly in the midgut of the insect, and eventually, parasite forms infective for mammals are discharged with the feces at the time of a subsequent blood meal. Transmission to a second mammalian host occurs when mucous membranes, breaks in the skin, or conjunctivas are contaminated with insect feces containing infective forms. The parasites can adhere to and penetrate various cell types in the new host and having done so multiply intracellularly. When the infected cell is overwhelmed by the multiplying parasites, it ruptures, releasing the organisms that then invade adjacent tissues and spread via the lymphatics and bloodstream to distant sites, where they go through further cycles of intracellular multiplication. As the parasites cycle asynchronously in this manner, lifelong parasitemias infective for vectors are maintained. T. cruzi can also be transmitted by transfusion of blood donated by persons who chronically harbor the parasite (4,5), from mother to fetus (6,7), and in laboratory accidents (1,8).

Epidemiology
Historically, Chagas disease has been a public health problem throughout Latin America, with the exception of the Caribbean nations. The Pan American Health Organization (PAHO) estimates that 8 million persons are chronically infected with T. cruzi and that 12,000 people die of the illness each year (9,10). Most persons who harbor the parasite chronically are unaware that they are infected, and only 10% to 30% will ever develop the manifestations of chronic clinical Chagas disease (11). The economic burden of Chagas disease is enormous, and its total annual cost in all endemic countries is estimated to be more than U.S. $8 billion (12). Considered from a global perspective, Chagas disease constitutes the third largest tropical disease burden, after malaria and schistosomiasis.
Despite the enormous social and economic burdens that result from the high overall prevalence of T. cruzi infection, the situation regarding current incidence rates of transmission is markedly brighter. In 1991, the countries of the Southern Cone of South America (Bolivia, Chile, Uruguay, Paraguay, Argentina, and Brazil) initiated an ambitious program aimed at interrupting the transmission of T. cruzi, called the Southern Cone Initiative (SCI) (13). Vector control through spraying of insecticides and housing improvement, education of persons at risk, and serologic screening of donated blood are the core elements of the program. During the first 8 years of the SCI, U.S. $340 million was invested in these efforts and a striking level of success was achieved. Ongoing surveillance shows clearly that transmission has been interrupted in vast areas of several endemic countries. Major reductions in T. cruzi prevalence rates in younger age-groups and a gradual decrease among persons who present for blood donation stand as clear evidence of the success of the initiative (14). Uruguay was certified free of transmission in 1997, Chile followed 2 years later, and Brazil was certified transmission-free in 2006. Moreover, during the past decade, programs similar to the SCI have been implemented in the Andean countries and in the nations of Central America, and major progress is being made in these efforts.
The epidemiology of T. cruzi infection in Mexico merits special mention because it has been studied less intensively there than in other endemic countries and because roughly 17 million persons born in Mexico now live in the United States (15). In a national survey among Mexican blood donors done in the mid-1990s, a 1.5% overall prevalence rate of T. cruzi infection was found, with the highest rates in the states of Hidalgo (2.8%), Tlaxcala (1.9%), and Puebla (1.8%) (16). In a study of T. cruzi infection among blood donors in the states of Jalisco and neighboring Nayarit, Kirchhoff et al. (5) found an overall prevalence rate of 0.8%. Importantly, four of nine recipients of blood products from T. cruzi–infected donors were found to be infected with the parasite. Clearly, serologic testing of blood donors in Jalisco and Nayarit should be performed, and the epidemiologic evidence available from other regions suggests that testing of blood donors throughout Mexico is appropriate. Unfortunately, at present, Mexico is the only one of the 22 Chagas-endemic countries that does not have a universal blood donor screening program for Chagas disease.
Even though the sylvatic cycle of T. cruzi is present in large areas of the western and southern United States, only seven cases of autochthonous transmission to humans here have been described (17). In addition, screening of U.S. blood donors for Chagas disease, which began in 2007 and to date has involved the testing of more than 40 million units, has only turned up 17 donors who appear to have acquired T. cruzi infection in the United States (18). In the past three decades, about 15 laboratory-acquired and imported cases of acute Chagas disease have been reported to the Centers for Disease Control and Prevention (CDC) but only one in the latter group occurred in a returning tourist (19). However, three instances of tourists returning to Europe from Latin America with acute T. cruzi infection have been described as well as a similar case in Canada (20).
The number of persons living in the United States with chronic T. cruzi infections, however, has increased markedly in recent decades. More than 23 million persons born in endemic countries currently reside in the United States, and it is noteworthy that almost all of the 6 million non-Mexican immigrants from Chagas disease–endemic nations now living here were born in countries where the prevalence of Chagas disease is higher than it is in Mexico. The CDC recently estimated that 300,000 persons with chronic T. cruzi infection currently reside in the United States (21). Before donor screening was implemented, seven cases of transfusion transmission of T. cruzi had been reported in the United States and Canada (22), and two additional instances were found in trace-back studies done after screening started (23). The confirmed rate of T. cruzi infection found in blood donors since the implementation of screening in 2007 has been about 1 in 13,300 (24). Transplantation of organs obtained from three chronically infected Latin American immigrants caused five cases of acute Chagas disease, one of which was fatal (25). Regarding congenital transmission, a reasonable estimate would put the number of infants born in the United States each year with congenital Chagas disease at 63 to 315, but only one such infant has been described (26). Several factors likely underlie the lack of additional reports of congenital transmission in the United States, but the low level of knowledge about Chagas disease among caregivers certainly plays a major role (27).
Diagnosis
Because there are no specific epidemiologic or clinical markers of Chagas disease, a definitive diagnosis of the illness must be made by laboratory studies. In acute disease, the diagnosis is parasitologic and the highly motile parasites often can be found by microscopic examination of wet preparations of blood or buffy coat. Parasites also can sometimes be found in the pellets of spun cerebrospinal fluid (CSF) (28). Polymerase chain reaction (PCR)–based assays are sensitive and specific in acute Chagas disease, but they are not used widely because of their complexity and because they are not available commercially (29,30). Hemoculture, which requires a special medium, is rarely useful for diagnosing acute Chagas disease because it is not widely available, requires several weeks to complete, and may have a sensitivity of only 50% to 70% even in the best of hands. In terms of making a specific diagnosis in patients with T. cruzi infection who may have cerebral involvement, my view is that brain biopsy is unlikely to be diagnostic if a careful search in blood and other fluids is negative.
Chronic Chagas disease is usually diagnosed by detecting specific immunoglobulin G (IgG) antibodies that bind to T. cruzi antigens. At present, at least two dozen serologic assays for Chagas disease are available commercially (31), and they are used widely for screening donated blood and clinical testing in the endemic countries and other areas to which at-risk persons have emigrated. As with all assays, the sensitivities and specificities are less than 100%, and false-positive results typically occur with specimens from patients with leishmaniasis, malaria, syphilis, and other parasitic and autoimmune diseases. World Health Organization (WHO) recommends that samples be tested in two assays based on different methodologies before diagnostic decisions are made (32).
Two tests have been approved by the U.S. Food and Drug Administration (FDA) for clinical testing in the United States (Chagas Kit, Hemagen Diagnostics, Inc., Columbia, MD; Chagatest Recombinante, Laboratorios Wiener, Rosario, Argentina). The Abbott ARCHITECT Chagas assay (33), which is an accurate chemiluminescence assay based on a mixture of four hybrid recombinant proteins, is not cleared in the United States but is approved and used widely for both donor and clinical testing in the majority of the Chagas-endemic countries. The Abbott PRISM Chagas assay (34), an automated chemiluminescence assay based on the same recombinant proteins employed in the ARCHITECT Chagas, and the Ortho T. cruzi ELISA Test System (Ortho-Clinical Diagnostics, Raritan, NJ), a parasite lysate-based enzyme-linked immunosorbent assay (ELISA) (35), are FDA-approved for donor testing and are currently used to screen the U.S. blood supply. A Clinical Laboratories Improvement Act (CLIA)–approved radioimmune precipitation assay (Chagas RIPA) (31,36) is employed for confirmatory testing of donated units that are repeat positive in the Abbott PRISM Chagas assay or the Ortho T. cruzi ELISA Test System and is available in my laboratory for testing research and clinical samples as well. Lastly, the Abbott ESA Chagas (enzyme strip assay), also based on the group of recombinant antigens used in the ARCHITECT Chagas assay, has been approved by the FDA for confirmatory testing of screen-positive donor samples (37). Unfortunately, PCR-based assays have not demonstrated consistently high levels of sensitivity that would justify their use for confirmatory testing of screen-positive donor samples (30).
Pathology and Clinical Manifestations
Acute and Indeterminate Phases of Chagas Disease
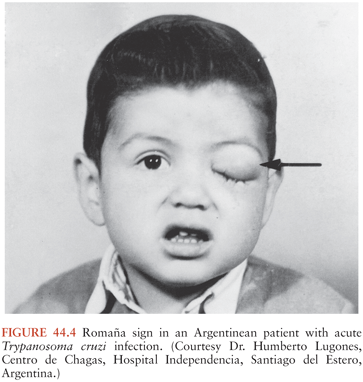
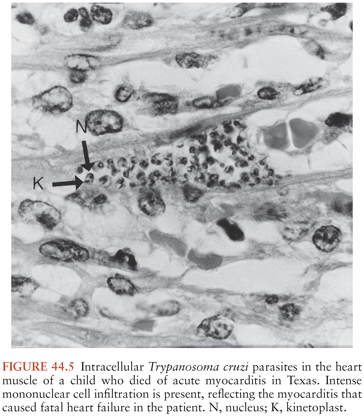
A chagoma, which is an erythematous and indurated lesion at the site where the parasite entered the host 10 to 14 days earlier, can be the first sign of acute T. cruzi infection (38). If the organisms enter through a conjunctiva, the patient may develop unilateral painless periorbital edema, which is called Romaña sign (Fig. 44.4). In any case, local histologic changes include intracellular parasitism of myocytes and various other cells, lymphocytic infiltration, interstitial edema, and reactive hyperplasia of adjacent lymph nodes. Spread of the parasites from the site of initial multiplication may be accompanied by fever and malaise, as well as edema of the face and lower extremities, hepatosplenomegaly, and widespread lymphadenopathy. Some patients with acute Chagas disease develop morbilliform rashes called schizotrypanides. Muscles can become heavily parasitized, and clinically significant myocarditis occurs in a small percentage of patients, occasionally resulting in fatal congestive heart failure (39,40) (Fig. 44.5). On histologic examination of cardiac tissue of such patients, patchy areas of infected cells are present, as are intense infiltration of mononuclear cells and necrosis. The pathognomic pseudocysts also often observed are aggregates of T. cruzi amastigotes inside cardiomyocytes. Nonspecific electrocardiographic (EKG) abnormalities may be present, but the life-threatening dysrhythmias that often are part of chronic cardiac Chagas disease usually do not occur.
Patients with acute Chagas disease can also have CNS involvement, which was first described by Carlos Chagas (2). How often the parasites actually invade the CNS in acutely infected patients is not known, but in general, neurologic findings are uncommon. It is noteworthy that in one study, parasites were found in the CSF of 8 of 11 patients examined, but none had neurologic symptoms (28). Meningoencephalitis is a rare occurrence in acute Chagas disease, and it is associated with a poor prognosis (41). It usually occurs in the first few months of life. At a cellular level, focal presence of parasites accompanied by mononuclear cell infiltration has been described in all anatomic areas of the brain. Most of the parasites are inside macrophages and glial cells, and parasitized neurons are rare. The clinical manifestations of meningoencephalitis in patients with acute T. cruzi infection are indistinguishable from those associated with other infectious agents that similarly affect the CNS. Seizures occur commonly in variable patterns, and spasticity and hyperreflexia are often present.
The signs and symptoms of acute Chagas disease resolve spontaneously in 4 to 8 weeks in almost all patients, and they then enter the indeterminate phase of the T. cruzi infection. This phase is characterized by lifelong subpatent parasitemias, a lack of symptoms, and generally detectable antibodies to T. cruzi antigens.
Chronic Chagas Heart Disease
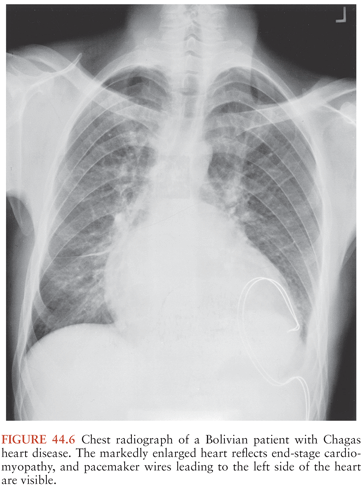
Fortunately, only 10% to 30% of persons with long-standing T. cruzi infections ever develop symptomatic chronic Chagas disease. Signs and symptoms typically first appear years or even decades after initial infection. Cardiac problems are the most common result of long-term T. cruzi infection, and during the past decade or so, convincing evidence has accumulated indicating that the persistence of parasites in heart muscle stimulates a chronic inflammatory process that leads to organ dysfunction and, in many cases, death (42,43). The inflammatory process can lead to various cardiac rhythm disturbances, including atrial fibrillation and atrial bradyarrhythmias; premature ventricular contractions; bundle–branch blocks, often of the right bundle; and complete atrioventricular block. These abnormalities can cause dizziness and syncope, and sudden death is common (44). Fibrosis, cardiomyopathy, and biventricular dilation can also develop, resulting in congestive heart failure, clot formation, and strokes (Fig. 44.6).
Heart transplantation is an option in persons with severe Chagas heart disease, and well more than 100 T. cruzi–infected patients have had this procedure in Brazil and the United States (45,46). Not surprisingly, T. cruzi has been found in endomyocardial biopsies of transplant recipients, thus raising the specter of early destruction of the new hearts by invasion of parasites. Somewhat surprisingly, however, long-term survival is greater in patients with Chagas disease who have undergone cardiac transplantation than in patients transplanted for heart diseases of other etiologies. It is also noteworthy that patients who have had transplants for Chagas heart disease often develop skin lesions containing high numbers of intracellular parasites. It is interesting to note that neither cerebral abscesses nor encephalitis due to T. cruzi reactivation has been noted in patients with Chagas disease after heart transplantation, although this commonly occurs in HIV-infected patients in whom chronic T. cruzi infection reactivates.
Chronic Gastrointestinal Chagas Disease (Megadisease)
The gastrointestinal (GI) tract is the second organ system most frequently affected in patients with chronic T. cruzi infection. Symptoms caused by megaesophagus are the most typical clinical manifestations of megadisease, although problems related to megacolon are common as well. Patients with megaesophagus have complaints similar to those of idiopathic achalasia such as dysphagia, odynophagia, cough, regurgitation, and chest pain (47,48). Hypersalivation and parotid gland hypertrophy have been observed as well. Aspiration can occur, and repeated episodes of aspiration pneumonitis are common in patients with severe, untreated, esophageal dysfunction. Malnutrition can combine with pneumonitis to cause death in patients with megaesophagus, although this is not common today.
Patients with megacolon usually complain of abdominal pain and chronic constipation. Patients with advanced megacolon can go for weeks between bowel movements, and acute obstruction, sometimes associated with volvulus, can result in perforation, septicemia, and death (49).
Trypanosoma cruzi Infection, Immunosuppression, and HIV
When patients with chronic T. cruzi infections become immunosuppressed, reactivation can sometimes occur in a manner and with a severity not seen in immunocompetent persons (50–53). In general, reactivation occurs in a few patients with chronic T. cruzi infection who become immunosuppressed, but the precise incidence is not known. Immunosuppression is the only clinical context in which brain abscesses and encephalitis occur in persons with chronic T. cruzi infections. Several reports of reactivation after kidney transplantation have appeared, and in rare cases, CNS abscesses were involved (54–56). In terms of organ donors with chronic T. cruzi infection, all otherwise acceptable organs should be considered for transplantation, with the exception of the heart and segments of the GI tract. After transplantation, however, patients receiving such organs should be monitored closely for acute Chagas disease with guidance from CDC staff.
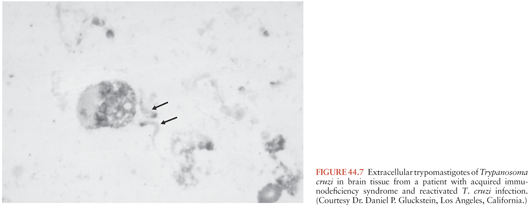
Reactivation of T. cruzi infections and associated CNS mass lesions or encephalitis has been reported in patients who become immunosuppressed in the course of other illnesses. In one patient, a tumor-like cerebral lesion due to T. cruzi was discovered at the same time chronic lymphocytic leukemia was diagnosed (57). Much more common, however, is the occurrence of T. cruzi reactivation in persons co-infected with T. cruzi and HIV, dozens of whom have been described (58–60). A large proportion of these patients developed CNS mass lesions due to T. cruzi (Fig. 44.7), which as noted simply do not occur in immunocompetent persons with chronic Chagas disease. Myocarditis also has been noted to occur commonly in dually infected persons in whom T. cruzi reactivates.
Treatment
In general, the two drugs available for treating patients with T. cruzi infection are unsatisfactory from several perspectives (61). The first of these, the nitrofuran derivative nifurtimox (Lampit, Bayer 2502), has been in use for more than four decades. In acute and congenital Chagas disease, nifurtimox reduces the duration and severity of the illness and decreases mortality. Unfortunately, it results in parasitologic cure only in approximately 70% of acutely infected patients. Therapy with nifurtimox should be initiated as early as possible in cases of acute or congenital Chagas disease. A large percentage of patients treated with nifurtimox experience bothersome side effects (62). GI complaints include anorexia, nausea, vomiting, abdominal pain, and weight loss. Possible neurologic problems include insomnia, restlessness, twitching, and paresthesias. These symptoms generally disappear when the dosage is reduced or treatment is stopped. In the United States, nifurtimox is only available from the CDC Drug Service.
Benznidazole (Rochagan, Roche 7-1051), a nitroimidazole derivative, is the second drug used to treat Chagas disease (63). Its efficacy is similar to that of nifurtimox. Considerable experience has accumulated with the use of benznidazole to treat babies with congenital T. cruzi infections during the first year of life. Cure rates are thought to be close to 100% in these patients. Side effects of benznidazole include granulocytopenia, peripheral neuropathy, and rash (64,65). Benznidazole is viewed as the drug of choice by most specialists in Latin America, where it is used widely. It also must be obtained from the CDC Drug Service for use in the United States. The regimens of nifurtimox and benznidazole used to treat patients with CNS Chagas disease are the same as those used in persons with other forms of T. cruzi infection.
Regarding which groups of T. cruzi–infected persons should be given specific therapy, the consensus of experts is that babies with congenital Chagas disease, all persons with acute T. cruzi infection, and chronically infected children up to 18 years old should receive specific treatment. Clinical trial data indicate clearly that a large proportion of patients in these groups who are given treatment are cured parasitologically (66–68). The question of whether persons in the indeterminate or chronic symptomatic phases of Chagas disease should be given nifurtimox or benznidazole has been debated for decades. This is a difficult question because these two drugs have to be taken for 2 to 4 months, often cause bothersome side effects, and in persons with long-standing T. cruzi infections may have parasitologic cure rates of only about 10% (69–71). In addition, there is no convincing evidence from properly controlled trials that full courses of either of the drugs improves long-term outcomes in patients with chronic infection (72). The issue is further complicated by the lack of sensitive and specific tools for determining if treated patients are cured parasitologically. A panel of experts assembled at the CDC in 2006 suggested that therapy be offered to persons aged 19 to 50 years with long-standing indeterminate phase infections (68). A major randomized controlled trial currently is being performed in Colombia, Brazil, and Argentina (the BENEFIT Multicentric Trial) to assess clinical outcomes in patients treated with benznidazole (73).
HUMAN AFRICAN TRYPANOSOMIASIS (SLEEPING SICKNESS)
Life Cycle and Mechanisms of Transmission
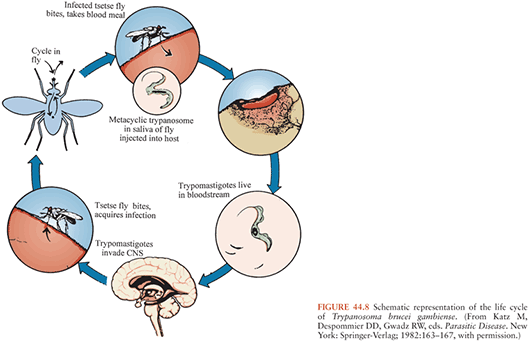
As noted earlier, HAT is caused by the two trypanosome subspecies T. brucei rhodesiense and T. brucei gambiense, which cause East African and West African trypanosomiases, respectively but are indistinguishable morphologically (Figs. 44.8 and 44.9). These organisms are members of the T. brucei complex and are transmitted by several species of tsetse flies that belong to the genus Glossina (74,75), which are found only in Africa. Tsetse flies of both sexes become infected with trypanosomes when they ingest blood from infected mammalian hosts. The ingested organisms go through a developmental cycle in the GI tract of the insects and then migrate to the salivary glands. The cycle of transmission is completed when infective forms are inoculated with saliva of the insect during a subsequent blood meal and begin multiplying in the new host, eventually invading the bloodstream and other extracellular spaces (Fig. 44.10). Essentially all transmission of African trypanosomes among both domestic and wild animals, as well as to humans, occurs in this cyclic manner. Mechanical transmission by vectors may occur, but it is not thought to be important. Transmission by blood transfusion and congenital transmission in humans are extremely rare (76,77). Laboratory accidents resulting in infection with African trypanosomes have been reported (8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree