VACCINES AGAINST BACTERIAL MENINGITIS
AMANDA C. COHN AND NANCY E. MESSONNIER
Three major pathogens—Haemophilus influenzae type b (HIB), Neisseria meningitidis (meningococcus), and Strep tococcus pneumoniae (pneumococcus)—cause the majority of bacterial meningitis cases in the United States and globally each year (1). Prior to implementation of vaccines targeting these causes of meningitis, these three agents constituted more than 75% of all cases of bacterial meningitis in most studies and 90% of bacterial meningitis in children (2–4). Current vaccines have virtually eliminated HIB meningitis and drastically reduced the burden of pneumococcal and meningococcal meningitis.
HAEMOPHILUS INFLUENZAE TYPE B
Before the introduction of effective vaccines, HIB was the leading cause of bacterial meningitis in children younger than 5 years in the United States. Approximately 120,000 cases of HIB meningitis and 7,500 cases of other invasive HIB infections occurred each year, and 1 in 200 children developed invasive HIB disease by 5 years of age (5,6). In Europe, Latin America, and Africa, annual rates of HIB disease varied from 20 to 60 per 100,000 children younger than 5 years prior to introduction of HIB vaccines (4,7,8). The case-fatality proportion for HIB was 2% to 5%, and 15% to 30% of survivors of meningitis had hearing impairment or other neurologic sequelae (5). In some developing countries, case-fatality ratios of 40% have been reported (9).
In industrialized countries, HIB most commonly presented as meningitis; in the United States before widespread vaccination, 60% to 70% of invasive disease due to HIB was associated with meningitis. Epiglottitis and bacteremia without focus were the next most common presentations (10). In contrast, in developing countries, the primary manifestation of HIB disease is lower respiratory tract infection. HIB infection may account for 5% to 8% of all pneumonia in children in these areas (11). In the developing world prior to implementation of HIB vaccines, H. influenzae infection (including all serotypes and nontypeable strains) was estimated to cause 482,000 pneumonia-related deaths each year among children younger than 5 years (12).
In the absence of vaccination, the highest rates of HIB disease occur in children younger than 2 years, but within that age-group, rates vary (10). In many European countries and in the general U.S. population, only 5% to 15% of cases occur before 6 months of age (10). In Native Americans and children in many developing countries, 30% to 40% of cases occur before 6 months of age (4,13). In addition to age, risk of HIB in the United States was increased among Alaskan natives and Navajo and Apache Indians, which may be because of host factors, including genetic susceptibility, as well as environmental factors. Blacks and Hispanics were also at higher risk, which was thought to be caused by nongenetic risk factors, such as low socioeconomic status, crowding, and large family size (10).
Microbiology, Immunology, and Pathogenesis
H. influenzae are gram-negative coccobacilli that are divided into unencapsulated (nontypeable) and encapsulated strains. The encapsulated strains are further classified into serotypes a through f, based on the antigenic characteristics of their polysaccharide capsules.
Humans are the only known biologically important reservoirs of H. influenzae, which is transmitted through direct or indirect exchange of oropharyngeal secretions. After introduction into the oropharynx, H. influenzae can establish colonization, cause lower respiratory tract infection, or invade through the nasal mucosa to enter the bloodstream.
The remarkably successful HIB conjugate vaccines developed in the 1980s originated from investigations begun in the 1930s. Pittman (14) and others showed that although six types of polysaccharide (types a through f) make up the capsules of invasive strains of H. influenzae, virulence was especially associated with type b, later characterized as polyribosyl-ribitol-phosphate (PRP). Also in the 1930s, it was shown that serum bactericidal antibodies directed against the capsular polysaccharide were protective. Infants and children lacked these antibodies and hence were prone to the disease, in contrast to older age-groups in whom HIB infection was rare. In the 1940s, absorption with purified type b polysaccharide was shown to remove the protective activity from rabbit hyperimmune serum (15), but it was not until the 1970s that anticapsular antibodies were proven capable of preventing disease (7).
Haemophilus influenzae Type B Polysaccharide Vaccines
In the 1970s, the ease of isolating and purifying PRP from the bacterial capsule prompted researchers to try it as a vaccine. The first trials in adults were encouraging; PRP was safe and elicited a good antibody response in adult vaccinees (16). The results in children, however, were disappointing because PRP is a T-cell–independent antigen and is poorly immunogenic in infants and young children. Among children 18 to 71 months of age in Finland, one dose of PRP vaccine was effective, but it was ineffective in infants 3 to 17 months of age (7). The conclusion from this trial was that 60% of all invasive HIB infections in Finland might be preventable by routine vaccination when children reached the age of 18 months. Based on the Finnish data, in 1985, the United States approved PRP vaccines for children 18 to 59 months of age. During the following 5 years, more than 10 million doses were administered (17), and the vaccines proved very safe with no serious adverse reactions reported (18).
However, reports of vaccine failures soon appeared and postlicensure case–control studies raised questions about the efficacy of HIB polysaccharide vaccines in clinical practice. In five of eight studies, estimated vaccine efficacy rates ranged from 41% to 88%, but three studies estimated that efficacy was less than 0% (19). There has not been a full explanation for these variations. Although a vaccine that prevented even half of invasive HIB disease may have been useful, these vaccines were clearly suboptimal, especially among children younger than 18 months, who were at greatest risk of disease. Vaccination studies using PRP also showed that this vaccine did not prime for immunologic memory or prime cells for subsequent booster response to additional doses (20), a problem in infants who do not respond well to polysaccharide antigens. However, these PRP vaccine studies were useful precursors for the development of next-generation vaccines.
Haemophilus influenzae Type B Conjugate Vaccines
Conjugate vaccines opened a new era in the prevention of HIB meningitis and other HIB diseases. Persons immunized with a conjugate vaccine develop higher concentrations of anti-PRP antibodies, and these vaccines are immunogenic in young children and induce a rapid booster response in persons who are reexposed to the antigen. Although major differences exist in the physicochemical structure and properties of the available HIB conjugate vaccines, the basic principle is the same for all HIB conjugate vaccines. Each conjugate vaccine consists of a carrier protein covalently conjugated to PRP or parts of the PRP molecule with or without a linker, converting PRP from a T-cell–independent antigen to a T-cell–dependent antigen. The HIB vaccines represented the first application of this important concept for the production of vaccines, which was subsequently applied to N. meningitidis and S. pneumoniae.
Immunogenicity and Safety
Not surprisingly, given the differences between the various HIB conjugate vaccines, the immunogenicity of these vaccines differs. In general PRP-D (diphtheria toxoid conjugate), HbOC (nontoxic variant of diphtheria toxoid conjugate), PRP-OMP (meningococcal outer membrane protein conjugate), and PRP-T (tetanus toxoid conjugate) behave similarly, each requiring two or three doses to reach substantial antibody levels (21). Data from the Finnish PRP efficacy trial was analyzed to provide estimates that an anti-PRP antibody concentration of 0.15 µg/mL obtained 1 month after vaccination was associated with short-term protection and that a postimmunization peak of 1.0 µg/mL indicated protection for up to 1 year (22). In one of the few studies in which three doses of all four preparations were administered in a double-blind and randomized fashion, more than 90% of vaccines exceeded the mean PRP antibody concentration of 0.15 µg/mL after vaccination with PRP-T, HbOC, or PRP-OMP at 1 month after vaccination, whereas only 58% of vaccinees exceeded this level after vaccination with PRP-D. A concentration of 1.0 µg/mL was reached by 83% of PRP-T vaccinees, 75% of HbOC vaccinees, 55% of PRP-OMP vaccinees, and 29% of PRP-D vaccines (21). Recipients of PRP-OMP, in contrast to recipients of PRP-D, HbOC, and PRP-T, developed high antibody concentrations after the first dose, even in 2-month-old infants. Administration of a second dose recruits a small percentage of additional responders, but a third dose adds little (23). However, PRP-OMP vaccination does not lead to as high a peak in antibody concentration after a full course, as is achieved after vaccination with HbOC or PRP-T vaccines (23). Antibody levels decrease over time after administration of the primary series of each HIB conjugate vaccine. Because a person immunized with a conjugate vaccine should respond with a rapid anamnestic antibody production, even though serum antibodies are no longer detectable, the boost response may protect the individual on exposure.
These vaccines have been used widely since 1990, and adverse reactions are generally uncommon. The most common reactions are local irritation, erythema, and pain, which have been reported in 5% to 30% of recipients and usually resolve within 12 to 24 hours. Systemic reactions such as fever and irritability are uncommon (24,25).
Efficacy and Impact
In the United States, following demonstration of their superior immunogenicity compared with HIB polysaccharide vaccines, three HIB conjugate vaccines were licensed between 1987 and 1989, and in April 1990, universal vaccination of children beginning at 15 months of age with PRP-D, HbOC, or PRP-OMP was recommended by the Advisory Committee on Immunization Practices (ACIP). In October 1990, following prospective studies showing good efficacy in infants, universal immunization of infants starting at 2 months of age with an HIB conjugate vaccine was recommended by ACIP (26,27). PRP-T is given as a three-dose primary series at 2, 4, and 6 months, with a booster dose at 12 to 15 months. PRP-OMP is given as a two-dose primary series at 2 and 4 months, with a booster dose at 12 to 15 months. These vaccines can be used interchangeably, but if PRP-T is used for either of the first two doses, a three-dose primary series should be used. HIB conjugate vaccines not only are immunogenic and efficacious, but they also reduce nasopharyngeal carriage of HIB among vaccinated children, decreasing transmission of H. influenzae and resulting in herd immunity (13,28,29). This important benefit is probably due to the high concentration of antipolysaccharide antibody, induced by exposure to the organism or bacteria exhibiting cross-reactive antigenicity, which results in transudation of immunoglobulin G (IgG) antibodies through the mucosal membranes. Vaccination does not seem to alter nasopharyngeal carriage rates of the other types of Haemophilus strains or pneumococci. Some studies suggest that the ability of HIB conjugate vaccines to reduce carriage may wane over time (29), but at least one study has shown persistent reduction in HIB carriage among children vaccinated as infants who had reached 4 to 5 years of age (30).
No other bacterial vaccine has had such widespread success over such a short period. Large-scale vaccinations were first launched in Finland in 1986; within 5 years, HIB meningitis and most other invasive HIB diseases were virtually eliminated (Fig. 51.1) (31). This remarkable decline in incidence occurred despite that during the first 2 years, 1986 and 1987, 50% of infants were vaccinated with PRP-D, the vaccine with the lowest immunogenic potential (21). In the United States, HIB disease decreased dramatically between 1988 and 1990, with increasing use of HIB conjugate vaccines, even among children younger than 1 year in whom vaccine was not being used (17). In 1997, the proportion of infants who had received HIB vaccine by age 7 months was only 61% (32), but rates of disease in children younger than 5 years decreased 99% because of the unexpected benefit of herd immunity (32). In the United States, HIB infection remains uncommon, with the highest incidence among Native Americans (33). However, 30 to 50 cases occur each year among infants younger than 6 months who have not completed the two- or three-dose primary series of HIB vaccination and among unvaccinated or undervaccinated children; primary or secondary vaccination occurs less frequently than failure to vaccinate.
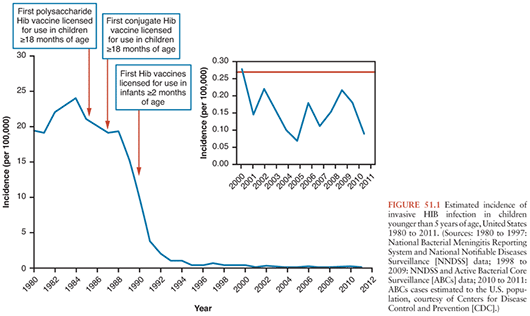
Not only has use of HIB conjugate vaccines reduced disease in children, but HIB disease in persons older than 5 years has also decreased, which has been attributed to the reduction of nasopharyngeal carriage and transmission of HIB in infants and children (31). The unexpected, dramatic impact of HIB conjugate vaccines on carriage raised concern that lowered nasopharyngeal competition from HIB would result in increased disease due to other serotypes and nontypeable H. influenzae, which are more common in adults than young children and are thought to be less virulent. Although the reduction in HIB disease increased the proportion of H. influenzae infection caused by other serotypes, widespread increases in disease caused by other serotypes of H. influenzae have not been reported (34). However, in Alaska, among individuals 10 years and older, infection with other H. influenzae serotypes increased from 0.5 per 100,000 persons per year (1980 to 1990) to 1.1 per 100,000 persons per year (1991 to 1996) (35). However, this rate has stabilized with no further increases since that time.
Control of Haemophilus influenzae Type B Infection
Close contacts of patients with HIB have higher rates of nasopharyngeal colonization with HIB. In susceptible individuals, invasive disease may follow colonization. Studies in the 1970s demonstrated that the risk of secondary disease was elevated among children who are household contacts of an index case and daycare center contacts (36,37). In the 1980s, chemoprophylaxis with rifampin was recommended to prevent secondary disease among close contacts. Because widespread use of HIB conjugate vaccines has decreased the number of individuals susceptible to invasive HIB disease, chemoprophylaxis is now indicated only if all contacts younger than 4 years are not fully vaccinated against HIB disease (27).
Most industrialized countries have now successfully implemented routine childhood immunization with HIB conjugate vaccines, with near elimination of HIB disease, a public health success story. In several countries in Europe, including Finland, instead of three doses, only two doses are administered primarily at 4 and 6 months of age with a booster at 14 to 18 months. This policy has proven successful; the incidence of HIB disease in Finland declined dramatically from 1988 to 1990. Germany, Switzerland, Austria, and Ireland have followed the same policy with similar favorable results. The United Kingdom chose another protocol in which no booster is administered, based on the finding that antibody concentrations persisted long after an accelerated vaccination schedule. Three doses of HIB conjugate vaccine were given at 2, 3, and 4 months of age (38). However, based on slowly increasing rates of HIB disease in children who did not receive a dose of HIB vaccine after age 12 months, in 2003, the United Kingdom added an HIB booster dose at age 12 months.
In the United States, previously unvaccinated infants aged 2 to 6 months receive three doses of PRP-T administered 2 months apart or two doses of PRP-OMP, followed by a booster immunization at age 12 to 15 months, at least 2 months after the last vaccination (27). HbOC is no longer manufactured. In general, vaccines from different manufacturers that protect against the same disease may be administered interchangeably in sequential doses in the immunization series for an individual patient (39). If PRP-OMP is combined with PRP-T, the recommended number of doses to complete the series is determined by the PRP-T product, not by the PRP-OMP vaccine. Among rural Alaskan native children, a change from PRP-OMP to HbOC in early 1996 was accompanied by an increase in HIB disease and in nasopharyngeal carriage of HIB, raising concern that use of HbOC vaccine left partially vaccinated children susceptible to ongoing HIB transmission that had not been eliminated by PRP-OMP (40).
Despite the availability of HIB conjugate vaccines since the 1980s and the virtual elimination of HIB in most i ndustrialized countries, HIB vaccine has only recently been introduced in developing countries where the burden of HIB morbidity and mortality is considerably higher. Prior to 2002, only 2 of 51 countries with an infant mortality rate more than 70 per 1,000 livebirths were routinely vaccinating against HIB—the Gambia and Bolivia. The major obstacles to the introduction of HIB conjugate vaccine into developing countries have been cost and the unrecognized burden of HIB disease. In 2000, the Global Alliance for Vaccines and Immunization (GAVI) and the affiliated Vaccine Fund have provided funding for the poorest countries to obtain HIB vaccine (www.vaccinealliance.org/home/index.php). However, uptake was slow due in part to a lack of understanding of the full burden of HIB disease in these countries. In 2006, World Health Organization (WHO) recommended that HIB vaccines should be included in every national immunization program. By 2009, 60 low-income countries had introduced HIB vaccines with GAVI support, preventing an estimated 430,000 future deaths (41). A 2008 study estimated that, within 4 years of GAVI support helping Uganda to introduce HIB vaccine into the national vaccination programme (June 2002), the incidence of HIB meningitis declined by 85%, and by the following year, the number of cases fell to nearly zero (42). The impact of HIB vaccine introduction has also been demonstrated in other GAVI-eligible countries including Kenya, Malawi, and Senegal (43–45). With 160 countries having introduced in 2009, still only 55% of the world’s children have access to vaccine, mostly due to delays in a few large countries (41). Although significant progress has been made in the number of children globally who have access to HIB vaccine, continuous efforts are still needed to improve its global coverage.
NEISSERIA MENINGITIDIS
N. meningitidis or meningococcal disease, first reported centuries ago, remains an important cause of disease globally (46). Two main forms of meningococcal disease exist, meningococcal sepsis and meningitis, which may occur separately or together. The third most common clinical presentation is meningococcal pneumonia, occurring in 5% to 15% of patients (47,48). Approximately 10% of patients with meningococcal disease die despite optimal medical care, and meningococcemia, a fulminant form of sepsis, can cause death within a few hours (49). In addition, 11% to 19% of survivors have long-term sequelae including hearing loss, mental retardation, and loss of limbs (50,51). In industrialized countries, the overall incidence of meningococcal disease is relatively low at 0.5 to 3 cases per 100,000 per year; however, meningococcal disease is unique among causes of bacterial meningitis because it causes both endemic disease and epidemics. Because of the high case-fatality rate, substantial sequelae, and propensity to cause epidemics, vaccination is a major focus of meningococcal disease prevention.
Outside of the meningitis belt of sub-Saharan Africa, infants have the highest risk of meningococcal disease. Rates of disease decrease after infancy and then increase in adolescence and young adulthood. Although rates decrease after early adulthood, a substantial number of cases occur among adults 23 to 64 years of age (48). Rates increase again in the elderly (48). In addition to age, other individual risk factors for meningococcal disease include underlying immune deficiencies, such as asplenia, properdin deficiency, and a deficiency of terminal complement components (52,53). Crowding, low socioeconomic status, active or passive exposure to tobacco smoke, and concurrent upper respiratory tract infections increase the risk of meningococcal disease (54–56).
Organism characteristics also modulate the risk of disease. Meningococci associated with invasive disease elaborate a capsule. Meningococci can be divided into 12 serogroups based on their different capsular polysaccharides. Globally, more than 90% of cases are caused by the serogroups A, B, and C (57–60). In most European and many Latin American countries, serogroups B and C caused the majority of disease prior to vaccination with serogroup C conjugate vaccines, and serogroup A causes the majority of disease in Africa and Asia (57). In the United States, serogroup Y emerged in the 1990s as a substantial cause of meningococcal disease, but a significant increase has not been reported by other countries (47). Serogroups A, B, C, Y, W, and X are all capable of causing outbreaks, most characteristically serogroup A. Although epidemics of serogroup A meningococcal disease were common in industrialized countries a century ago, serogroup A epidemics now occur primarily in the African “meningitis belt,” a region of savannah that extends from Ethiopia in the east to Senegal in the west (61,62). In this region, endemic rates of disease are several times higher than in industrialized countries; in addition, major epidemics occur every 8 to 12 years, with incidence rates as high as 1% of the population, causing tremendous disruption to the already strained public health infrastructure (59). In 2000, the first documented epidemic of serogroup W occurred among pilgrims to the hajj in Saudi Arabia, and in 2002, the first major epidemic of serogroup W occurred in Burkina Faso (63). Since that time, serogroup W has remained in the meningitis belt and has been the cause of several outbreaks but does not tend to cause the explosive epidemics that are common with serogroup C disease.
In recent years, meningococcal conjugate vaccines have had a dramatic impact on the global burden of meningococcal disease. Meningococcal disease has a wide age distribution, and an effective vaccination program must address the risk of disease in infants as well as older children and adolescents.
Immunology and Pathogenesis
The human nasopharynx is the only natural reservoir of N. meningitidis, most of which are nonpathogenic and carried asymptomatically in 5% to 10% of adults (64,65). In less than 1% of those colonized, N. meningitidis penetrate the mucosa to gain access to the bloodstream and cause systemic disease (66).
In 1913, Flexner (67) demonstrated the efficacy of antimeningococcal serum in reducing mortality due to N. meningitidis, providing evidence of the importance of humoral immunity in protection. Except for serogroup B, the primary host defense against meningococci is circulating antibody against the capsular polysaccharide, as in HIB disease. In the neonatal period, natural resistance is due to the transplacental passage of IgG antibodies, which wane during the first 6 months of life; bactericidal antibodies are present in fewer than 25% of infants aged 6 to 23 months (68,69). As individuals develop antimeningococcal antibodies, their susceptibility to meningococcal disease decreases, which is further evidence of the role of humoral immunity. Nasopharyngeal colonization with meningococci and nonpathogenic Neisseria, such as Neisseria lactamica, a common commensal organism in infants and children, partially accounts for this immunizing process. However, a parallel rise in specific anti-A or anti-C capsular polysaccharide antibodies measured by radioimmunoassay cannot be explained solely by carriage of nonpathogenic Neisseria species, which do not express capsule (70,71). Therefore, natural immunization through unrelated but serologically cross-reactive bacteria, such as Escherichia coli K1, itself a major cause of neonatal meningitis, is likely (72).
History of Meningococcal Vaccines
The history of attempted immunization against meningococcal disease, originally known as cerebrospinal fever, is long. Whole-cell and autolysate vaccines were used well before the natural history and pathogenesis of the disease were fully understood. The correlation between susceptibility to disease and the absence of circulating bactericidal antibodies was not documented until 60 years later (69). Vaccination trials were carried out in the Sudan in 1915 and among U.S. and British troops during the cerebrospinal meningitis epidemics of World War I (61,73).
In the 1940s, encouraged by studies with pneumococcal polysaccharides in which a good seroresponse was achieved, the capsular polysaccharide of group A meningococci was purified using the same methods as for pneumococci and tested the product in human volunteers. The results were disappointing, and the study was neglected for two decades, until it was determined that the polysaccharide used was quite small, a potential cause of the poor immunogenicity. When Gotschlich, Liu, and Arenstein (73) modified the purification method, immunogenic preparations were obtained from both A and C strains, opening the way for a new generation of vaccines.
Meningococcal Polysaccharide Vaccines
By 1969, Gotschlich, Goldschneider, and Artenstein (74) demonstrated the immunogenicity of serogroup C vaccine in adults, and subsequent studies confirmed that immunogenicity was serogroup specific, so serogroup A vaccine does not protect against serogroup C. Because of the specificity of these vaccines, bivalent (A, C), trivalent (A, C, W), and quadrivalent vaccines (A, C, Y, W) have been developed (75,76). Prior to development of conjugate vaccines, bivalent and quadrivalent meningococcal polysaccharide vaccines were extensively used in mass vaccination programs and in the military. These vaccines are well tolerated with generally mild adverse events, most commonly pain and redness at the injection site lasting for 1 to 2 days. Transient fever occurs in 2% to 5% of vaccines, more commonly in infants (77). Severe adverse reactions, including systemic allergic reactions and anaphylaxis, are rare (77). Studies of vaccination during pregnancy have not documented adverse events among pregnant women or newborns (78,79).
Immunogenicity and Efficacy
Serogroup A and C meningococcal polysaccharide vaccines have consistently been found to have good immunogenicity and clinical efficacy in adults and children older than 5 years. Routine vaccination of military recruits in the United States has virtually eliminated meningococcal outbreaks in the military (80). In Italy, introduction of polysaccharide vaccines decreased rates of serogroup C meningococcal disease; vaccine efficacy was estimated as 90% (81). Similarly high efficacy has been demonstrated in community outbreaks of serogroup C meningococcal disease in Canada, Brazil, and the United States (82,83). For serogroup A, controlled trials and case–control studies have demonstrated efficacy rates of 85% to 90% after a single dose (70,84). Meningococcal polysaccharide vaccines have been shown to be effective in controlling meningococcal outbreaks in industrialized countries and Africa (82,83,85). Serogroup Y and W polysaccharides are safe and immunogenic in older children and adults; although clinical protection has not been documented, vaccination with these polysaccharides induces bactericidal antibodies (76,86).
Meningococcal polysaccharide vaccines do have some important limitations. The serogroup C polysaccharide does not induce good antibody response before 18 to 24 months of age (87). In healthy adult military personnel, anticapsular antibodies and bactericidal activity decrease substantially during the 2 years after vaccination but in some cases persisted for up to 10 years after immunization (88). In children 2 to 6 years of age at the time of vaccination, antibody levels decline consistently over 1 to 4 years (87). In infants immunized before 1 year of age, antibody levels decline to baseline after 3 to 5 months (77). In both children and adults, a significant response to a booster dose has not been observed. Although reduced clinical efficacy has not been demonstrated among individuals who have received multiple doses, studies have raised the question of immunologic tolerance to the serogroup C polysaccharide vaccine (89,90). Finally, polysaccharide vaccines do not completely protect from acquisition of nasopharyngeal carriage, and therefore, they do not provide long-term herd immunity.
Conjugate Meningococcal Vaccines
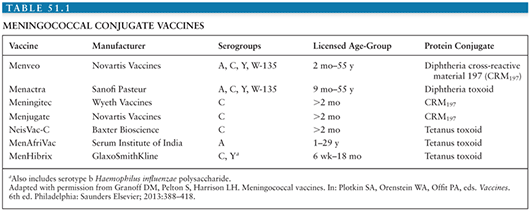
As with HIB and pneumococcal conjugate vaccines, serogroups A, C, Y, and W have been chemically conjugated to carrier proteins. Meningococcal conjugate vaccines induce a T-cell–dependent response that improves the immune response, especially in infants. Because rates of meningococcal disease are relatively low, clinical trials of vaccine efficacy are difficult; meningococcal conjugate vaccines have been licensed based on immunogenicity and safety data (Table 51.1).
Serogroup C Conjugate Vaccines
Meningococcal serogroup C (MCC) vaccines were introduced in the United Kingdom in 1999 in response to high rates of serogroup C disease caused by a virulent clone. After a mass vaccination campaign targeting ages 1 to 17 years, MCC was introduced into the routine infant immunization schedule. The current schedule is doses at age 3 and 4 months with a booster dose at 12 to 13 months. Canada and several countries in Europe have single-dose MCC recommendations in toddlers or multidose MCC schedules in infants.
The safety and immunogenicity of the three different MCC vaccines in children and adults have been demonstrated in several studies, and there are no substantial differences in effectiveness between the vaccine types (91–93). Ninety-eight percent of infants develop protective bactericidal antibody titers, following a three-dose series at 2, 3, and 4 months of age. Similar immune responses are seen following a two-dose series at 3 and 5 months as well as a single dose in older children and adults. Vaccine effectiveness in adolescents was 93% and sustained up to 4 years after the catchup vaccination (94). Although effectiveness was high (83%) in those receiving catchup immunization after 5 months of age, it was lower among those vaccinated in infancy (66%) (94,95).
Antibody persistence has been followed closely after MCC vaccination in the United Kingdom, and waning immunity is demonstrated, especially in infants and young children. In the absence of a booster dose, only 8% to 12% of children completing a three-dose series in infancy had protective immunity at age 4 years (96). Among children primed at ages ranging from 2 months to 6 years, only 25% had protective titers greater than or equal to 8 at 1 year (97). Waning immunity among adolescents vaccinated with MCC is much less pronounced. In two studies of adolescents vaccinated at age 10 years, 62% to 75% of adolescents vaccinated maintained protective antibody titers 3 to 6 years following vaccination (98,99). These studies suggest that immune maturation may play an important role in duration of protection.
Conjugate vaccine programs also have potential to provide indirect protection through herd immunity. Two years after introduction of MCC vaccine in the United Kingdom, serogroup C carriage was reduced by 67% (100). The proportion of nongroupable isolates increased (10.05% versus 11.2%, p = .071), but there was no significant change in carriage of N. meningitidis expressing other disease-associated serogroups (100). Attack rates among unvaccinated children in the United Kingdom also declined by 67% in the 4 years following vaccine introduction (94,95,101,102). Between 1998 and 2009, the incidence of serogroup C disease in persons older than 25 years dropped from 0.55 per 100,000 persons to 0.02 per 100,000 persons; and the number of cases in infants younger than 3 months of age dropped from 13 in 1998 to 1 in 2009 (102). These effects were seen despite a declining seroprevalence of protective antibodies among vaccination cohorts as early as 18 months after the last scheduled dose of vaccine, suggesting sustained protection due to herd immunity despite absence of protective antibodies in individuals.
Quadrivalent (Serogroups A, C, W, and Y) and Bivalent (Serogroups C and Y) Meningococcal Vaccines
In the United States, there are three licensed meningococcal conjugate vaccines: quadrivalent meningococcal conjugate vaccine (MenACWY) conjugated to diphtheria toxoid (MenACWYD) (Menactra, Sanofi Pasteur); MenACWY conjugated to CRM197, which is a nontoxic mutant diphtheria toxin (MenACWYCRM) (Menveo, Novartis); and bivalent meningococcal conjugate vaccine combined with HIB vaccine, conjugated to tetanus toxoid (HibMenCYTT) (MenHibrix, GlaxoSmithKline). MenACWYD is licensed as a single dose for persons ages 2 to 55 years and as a two-dose series for children 9 to 23 months. MenACWYCRM is licensed as a single dose for persons ages 2 to 55 years and a four-dose series in infants (103,104). HibMenCYTT, approved in 2012, is licensed as a four-dose series for infants aged 2 to 18 months. These vaccines all protect against serogroups C and Y, which cause approximately two thirds of cases in the United States. MenACWYD and MenACWYCRM also protect against serogroups A and W. Because of the frequency of meningococcal disease caused by serogroup Y in the United States, conjugate vaccines that do not contain serogroup Y are not licensed in the United States. Currently, adolescents are recommended to be vaccinated at ages 11 to 12 years with a booster dose at age 16 years; other persons at increased risk for meningococcal disease are recommended to be vaccinated as well. Routine immunization of infants in the United States is not recommended because currently licensed vaccines do not include serogroup B and because of a historically low burden of disease (107).
The duration of protection following vaccination with quadrivalent meningococcal conjugate vaccines remains an important subject of evaluation. Studies among adolescent recipients of MenACWYD and MenACWYCRM demonstrate significant declines in antibody levels over 5 years, which provides corroborating evidence for the observed decline in effectiveness discussed earlier. After a single dose of MenACWYD, the proportion of adolescents with protective antibodies was as low as 30% to 54% 3 years after vaccination (105). At 5 years postvaccination with MenACWYCRM, 34% (serogroup A) to 84% (serogroup W) maintained protective levels of antibody (106). Antibodies waned even faster in infants ages 9 to 23 months who received a two-dose series of MenACWYD (107). However, infants vaccinated with four doses of HibMenCY demonstrate better antibody persistence. Five years after the fourth dose, 82% and 69% of children immunized as infants had protective levels of antibody for serogroups C and Y, respectively (108). Estimates from a large ongoing case–control study evaluating one of the two MenACWY vaccines, MenACWYD, in the United States suggest high vaccine effectiveness early after vaccination, but 2 to 5 years after vaccination, vaccine effectiveness wanes to 50% to 60%. Waning immunity was the primary rationale for adding a booster dose for adolescents at age 16 years.
Serogroup A Conjugate Vaccine
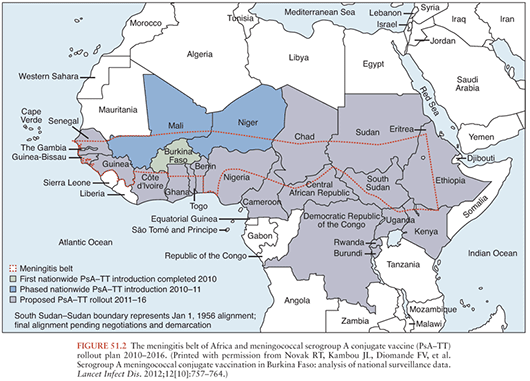
To address the devastating epidemics of serogroup A meningococcal (MenA) disease that occur in the meningitis belt of sub-Saharan Africa (Fig. 51.2), a MenA tetanus toxoid conjugate vaccine, called MenAfriVac (PsA-TT), was developed specifically for this region by a collaborative partnership, the Meningitis Vaccine Project. The goal of this project was to ensure a vaccine that would be available in the region that countries could afford to implement. In 2010, PsA-TT was licensed in 1- to 29-year-olds and in 2011 was prequalified by WHO for use in Africa. This is the first large-scale introduction of a meningococcal conjugate vaccine in Africa. At 40 cents per dose, the vaccine is priced so that mass vaccination of all 1- to 29-year-olds is affordable for the region.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree