Summary of key facts
- •
The concept of tumor immunosurveillance can be traced back to the early 1900s in association with observations that a potentially overwhelming frequency of carcinomas must be repressed by the immune system.
- •
The cancer immunosurveillance hypothesis was formally proposed in 1970 by Burnet and Thomas, , describing the immune contribution to tumor control as an “evolutionary necessity” given the lifetime accumulation of genetic changes in somatic cells.
- •
Despite setbacks from preliminary observations in the athymic nude mouse, a host of mouse models and epidemiologic data in the 1990s overwhelmingly supported the concept of cancer immunosurveillance.
- •
The cancer immunosurveillance hypothesis has evolved into the three Es of immune editing: elimination, equilibrium, and escape.
- •
Tumor-associated antigens can be recognized by the immune system and arise from multiple different processes, including mutation-associated neoantigens, reexpression or overexpression of antigens associated with immune privileged tissue, and posttranslational modifications, such as alternative splicing.
- •
Oncogene-induced senescence in the setting of intact tumor suppressor pathways can lead to antigen-specific immune responses that may contribute efficacy to tumor immunosurveillance and elimination.
- •
The microbiome can influence the function of innate and adaptive immune cells. In addition, given the genetic diversity of the microbiota, it is likely that resultant peptides will mimic neoantigens from tumors, representing a promising avenue to further understand the complexity of tumor immunosurveillance.
- •
Data from populations of transplant recipients and those with HIV infection demonstrate higher rates of cancer in individuals who are immunocompromised, supporting a role for the immune system in cancer prevention.
The concept of immune surveillance in cancer
Tumor immunology is grounded on the central principle that cancer can be controlled and even eliminated by an intact host immune response. Thus a resulting corollary is that cancer development and progression represent a failure, to some extent, of the immune system to perform one of its primary functions. This is a hotly debated topic historically, with large swings within the scientific community over the past few decades. In this manner, the history of tumor immunology highlights the importance of incorporating new insights into established concepts.
The concept of immune regulation in cancer development can be traced back to the works of Elie Metchnikoff and Paul Ehrlich, who received the Nobel Prize in 1908. Metchnikoff described the process of phagocytosis, laying the foundation for innate immunity. Ehrlich pioneered the idea of humoral immunity, and both scientists proposed that the immune system might control tumors. Ehrlich specifically suggested a potentially overwhelming frequency of carcinomas that must be repressed by the immune system. He hypothesized that this occurred via mechanisms outlined in the side-chain theory to describe soluble mediators and their receptors, analogous to side chains in dyes that determine coloring properties. ,
The contribution of cell-mediated immunity was expanded by Peter Medawar in the 1950s. The context of this advance was allograft rejection in transplantation. In fact, tumors transplanted within inbred strains of mice were not typically rejected, arguing against an immune-mediated antitumor response. However, certain tumor-associated antigens were confirmed to exist because these mice could be immunized against transplanted tumors, lending some support to the idea behind cancer immunosurveillance. The concept of cancer immunosurveillance was then formally proposed by Macfarlane Burnet and Lewis Thomas in 1970, which suggested that it was an “evolutionary necessity” for the immune system to control the lifetime accumulation of genetic changes in somatic cells.
The cancer immunosurveillance hypothesis underwent major setbacks over the following 2 decades because of a faulty assumption that any immune suppression would lead to higher rates of cancer. Experimental induction of an immunocompromised state was primitive at this time and typically involved thymectomy or antilymphocyte serum. Indeed, an increase was seen in these models in lymphomas and viral-associated tumors, but this was assumed to be because of infectious susceptibility rather than faulty immune surveillance against tumors. , The athymic nude mouse exploded onto the scientific scene during this time, bearing a spontaneous deletion in the Foxn1 gene that leads to thymic absence and a loss of mature T cells. These mice were first described by Flanagan in 1966 after their discovery within a group of albino mice in Glasgow. Nude mice became a commonly used strain because of their capacity to accept allografts and even xenografts without rejection.
A common model for spontaneous tumor induction at the time was 3-methylcholanthrene (MCA)-induced sarcomas. It was reasoned that if the cancer immunosurveillance hypothesis were true, then nude mice should develop more tumors with earlier onset after exposure to MCA. This was not the case in landmark experiments from Stutman and others, which led many investigators to conclude that the immune system does not affect cancer development. Rygaard and Povlsen went as far as performing necropsies on 10,800 nude mice between 3 to 7 months of age, with no detectable difference in tumor formation compared with immunocompromised mice, again concluding that the immune system did not play a role in cancer development. As an aside, nude mice have underdeveloped mammary glands and are unable to nurse their offspring, necessitating breeding with heterozygous females, lending some perspective to the scale of the work from these authors. Taking it a step further, Prehn uncovered inflammatory pathways that led to more aggressive tumors, ultimately proposing the immunostimulation theory to explain tumor-promoting inflammation. Taken together, these findings led many to believe that the immune system could not target spontaneously developing tumors and may even fuel tumor progression.
Mouse models stimulate resurgence of cancer immunosurveillance
To understand the gap between our current understanding of tumor immunology and the views held in the 1980s, it is worth examining the experimental limitations of this time. This is perhaps most evident in subsequent studies focusing on the biology of the athymic nude mouse. Not only do these mice have functional populations of T cells but they also produce a full complement of natural killer (NK) cells, about which little was known at the time. It has been subsequently shown that a completely functional innate immune system can maintain some degree of cancer immunosurveillance in conjunction with the incomplete adaptive immune system present in the nude mouse. It is worth noting that toxin metabolism also contributed to discrepancies between expected and observed MCA-induced tumor formation. The strain of control mice used by Stutman metabolized MCA into its carcinogenic form at a much higher rate than the nude mice, which predisposed these mice to higher rates of tumor formation. , Knowing these limitations, we can understand how the stage might be set for future insights to conflict with findings from the nude mouse.
Indeed, experiments incorporating MCA-induced tumor formation in mice on a different background (BALB/c) demonstrated increased tumor formation in athymic nude mice. The introduction of severe combined immunodeficiency (SCID) mice further supported the resurrection of the cancer immunosurveillance concept. These mice lack functional DNA-dependent protein kinase (DNA-PK) and are unable to undergo somatic recombination, resulting in a lack of functional B and T cells. SCID mice also demonstrated increased MCA-induced tumor formation, although critics at the time attributed increased tumorigenicity to global defects in DNA repair. ,
Definitive findings supporting tumor immunosurveillance are often attributed to a few sets of landmark tumor transplantation findings. The central concept here can be boiled down to this: If a tumor must evolve in response to cancer immunosurveillance to progress, these immune-evading adaptations may not be present in immunocompromised mice. Thus transplantation into an immunocompetent host may trigger immediate recognition and rejection. The field of cancer immunology would soon be inundated with investigations confirming this effect.
The effects of interferon gamma (IFN-γ) on immune stimulation and tumor immunosurveillance were soon confirmed. Mice with defects in IFN-γ signaling demonstrated enhanced MCA-induced tumorigenicity, and many of these tumors were rejected when transplanted into immune-competent hosts. A prominent role for cytotoxic T cells was confirmed with perforin −/− mice displaying enhanced susceptibility to tumor formation. The importance of lymphocytes was further confirmed with the development of recombination activating gene ( RAG) 1 or RAG2 knockout mice, genes required for somatic recombination and only expressed in lymphoid tissue. Thus the counterargument that global DNA repair defects led to cancer development no longer applied.
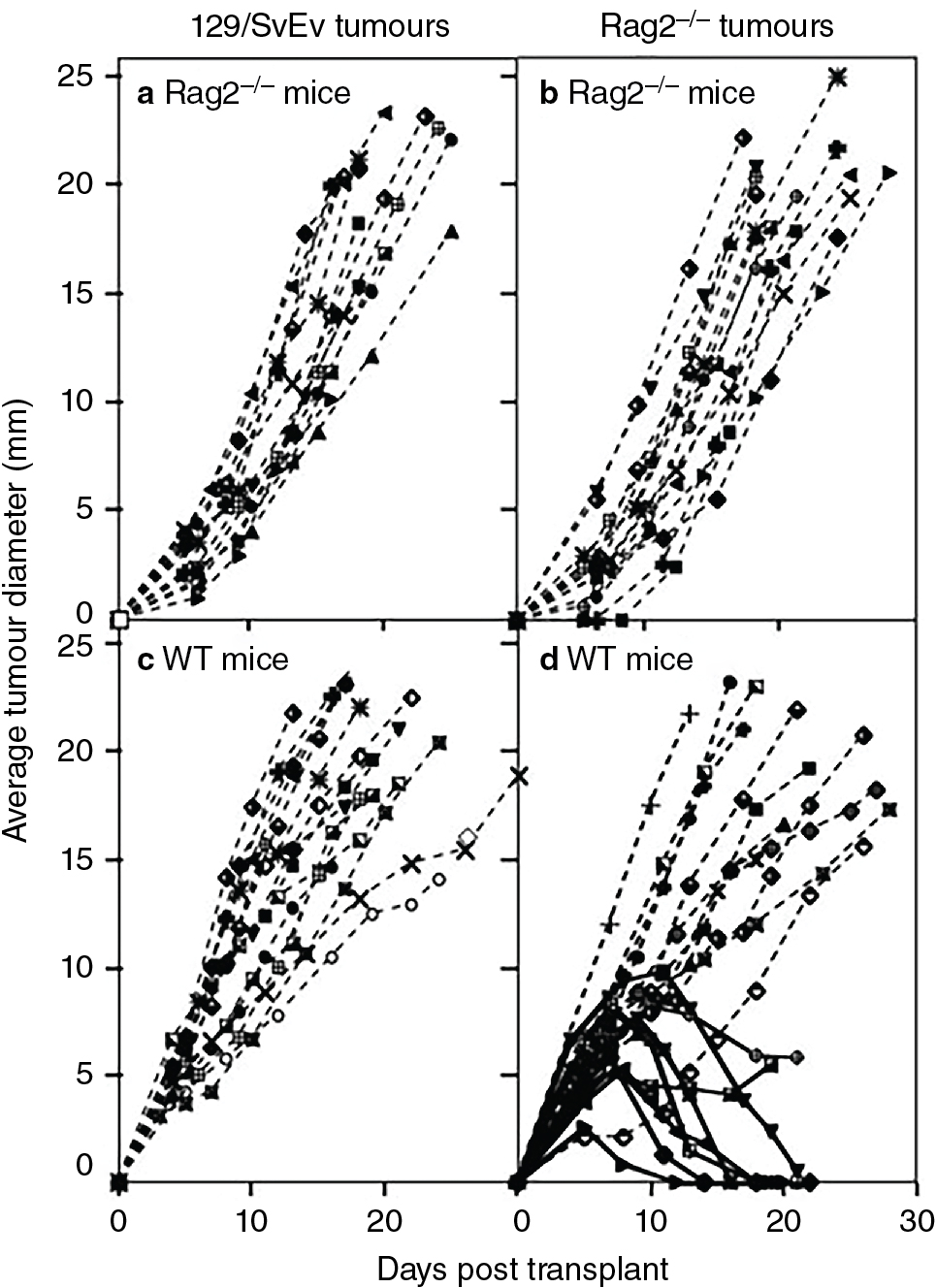
Schreiber et al. incorporated these concepts in a groundbreaking investigation that anchored support in the scientific community for the cancer immunosurveillance hypothesis. A key finding from these experiments is displayed in Fig. 6.1 . To summarize, mice with defects in RAG2 , IFNGR1 , STAT1 , or both RAG2 and STAT1 (RkSk) were all more susceptible to tumor formation from MCA treatment. Of note, STAT1 signaling is part of the IFN-γ pathway. Both RAG2 −/− and RkSk mice developed spontaneous tumors at a higher rate than controls in pathogen-free environments. Finally, approximately 40% of MCA-induced tumors transplanted from RAG2 −/− mice into immunocompetent controls were rejected. No tumors were rejected by RAG2 −/− mice transplanted from controls. A host of similar investigations supporting tumor immunosurveillance ensued, which are summarized in Table 6.1 . , , Thus advances in our understanding of mouse models effectively rejuvenated scientific enthusiasm for the tumor immunosurveillance hypothesis.
| Phenotype | Tumor Susceptibility |
|---|---|
| RAG2 −/− |
|
| RkSk |
|
| BALB/c SCID | MCA-induced sarcomas (Smyth et al, Int Immunol, 2001) |
| Perforn −/− |
|
| TCR Jα281 −/− | MCA-induced sarcomas (Shankaran et al, Nature, 2001), (Street et al, Blood, 2001), (Smyth et al, J Exp Med, 2000) |
| Anti-asialo-GM1 antibody | MCA-induced sarcomas (Shankaran et al, Nature, 2001) |
| Anti-NK1.1 antibody | MCA-induced sarcomas (Shankaran et al, Nature, 2001), (Smyth et al, J Exp Med, 2000) |
| Anti-Thy1 antibody | MCA-induced sarcomas (Shankaran et al, Nature, 2001), (Smyth et al, J Exp Med, 2000) |
| αβ T cell −/− | MCA-induced sarcomas (Girardi et al, Science, 2001) |
| γδ T cell −/− |
|
| STAT1 −/− | MCA-induced sarcomas (Shankaran et al, Nature, 2001), (Kaplan et al, PNAS, 1998) |
| IFNGR1 −/− | MCA-induced sarcomas (Shankaran et al, Nature, 2001), (Kaplan et al, PNAS, 1998) |
| IFN-γ −/− |
|
| Perforin −/− x IFN-γ −/− |
|
| IL-12 −/− | MCA-induced sarcomas (Smyth et al, J Exp Med, 2000) |
| Exogenous IL-12 | Lower incidence of MCA-induced sarcomas (Noguchi et al, PNAS, 1996) |
Current experimental evidence supporting tumor immune surveillance
The development of organ transplantation heralded a series of clinical observations confirming that patients who are immunocompromised are at higher risk for the development of cancer. Early epidemiologic studies examining this population did demonstrate an increase in cancers of viral etiology, such as lymphoma, Kaposi sarcoma, and anogenital carcinomas. Thus it was initially difficult to tease out whether viral susceptibility drove the increased rate of cancer, rather than defects in immunosurveillance. However, subsequent studies over longer periods of time confirmed significantly increased rates of malignant melanoma, particularly in the pediatric population. Investigations also demonstrated elevated rates of colon, pancreatic, lung, bladder, kidney, ureter, and endocrine tumors. , There is currently a wealth of data supporting increased rates of non-viral-associated malignancies in patients who are immunocompromised, lending further support to the cancer immunosurveillance hypothesis.
In addition to observations in individuals who are immunocompromised, the composition of immune cells within solid tumors has consistently demonstrated prognostic value, particularly with respect to cytotoxic T-cell presence. The concept of cancer immunosurveillance is perhaps most compellingly supported by the development of immune checkpoint blockade. Agents such as ipilimumab and nivolumab antagonize inhibitory receptors on T cells that accumulate with prolonged activation. These agents have met with unprecedented success in treating numerous types of tumors, confirming the idea that the immune system is capable of targeting tumors.
Immune editing in cancer
The concept of cancer immunoediting has evolved from the original hypothesis of cancer immunosurveillance to account for immune activity at later stages of cancer development. We can again draw on the experiments of Shankaran et al. for evidence of immune editing, which demonstrated that tumors formed in immunocompromised mice were more immunogenic that tumors formed in immunocompetent mice. A series of tumor transplantation experiments confirmed these findings. Clinical correlates supporting the concept of immune editing include frequent observations that tumors lack antigen presentation components or mediators of the IFN-γ signaling pathway.
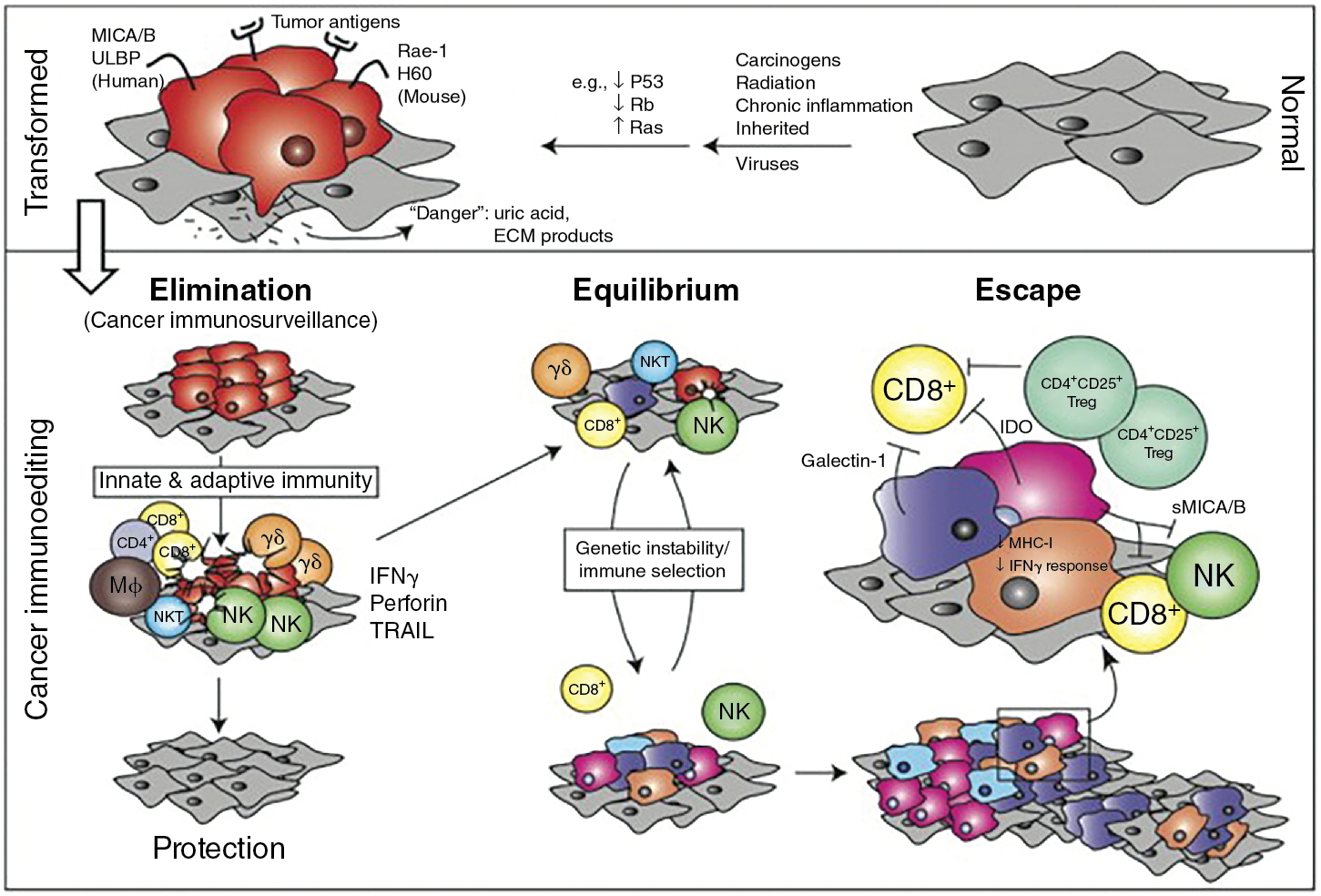
The current model of cancer immune editing encompasses three processes: elimination, equilibrium, and escape. Elimination is thought to include early alarm signals to the presence of a tumor, IFN-γ production, chemotaxis and activation of innate immune cells, limited tumor cell killing with antigen processing by dendritic cells, and, finally, T-cell activation and chemotaxis to eliminate the remaining tumor. In agreement with many of the experiments discussed in this chapter, IFN-γ is central to the process of early tumor elimination. However, the coordination of the innate and adaptive immune systems is also critical.
Equilibrium refers to a state of tumor development that has survived the elimination phase. Like the name implies, equilibrium can be likened to a stalemate between the immune system and the tumor. Lymphocytes and NK cells continue to exert selection pressure on the entrenched tumor as it continues to accumulate mutations and evolve in response to this pressure. The immune system is unable to eliminate the tumor, but the tumor is unable to progress. Equilibrium is thought to be the longest of the three processes, occurring over a period of years. One can imagine how this phase provides a perfect environment for tumors to evolve mechanisms to evade detection and/or killing by the immune system.
The escape process refers to the malignancies diagnosed clinically. By this point, the tumor has evaded constraints from the immune system and progresses at a largely unchecked pace. Until the 2000s, we had no reliable methods to stimulate the immune system and challenge this process. Immune checkpoint blockade has provided a way to even the balance again and confirm observations from a prolonged equilibrium phase, such as T-cell exhaustion with chronic stimulation. A summary of immune editing is presented in Fig. 6.2 . For further reading, the evolution of tumor immunosurveillance and current framework of immune editing is summarized well in Dunn et al.
Tumor-associated antigens
The idea of conserved tumor-associated antigens that provide targets for antitumor immune responses has been posited for some time. Early experiments in the 1960s demonstrated that irradiated tumor cells could induce sustained immunity to future tumor challenges. , Modern approaches have allowed us to identify many of these antigens at the molecular level.
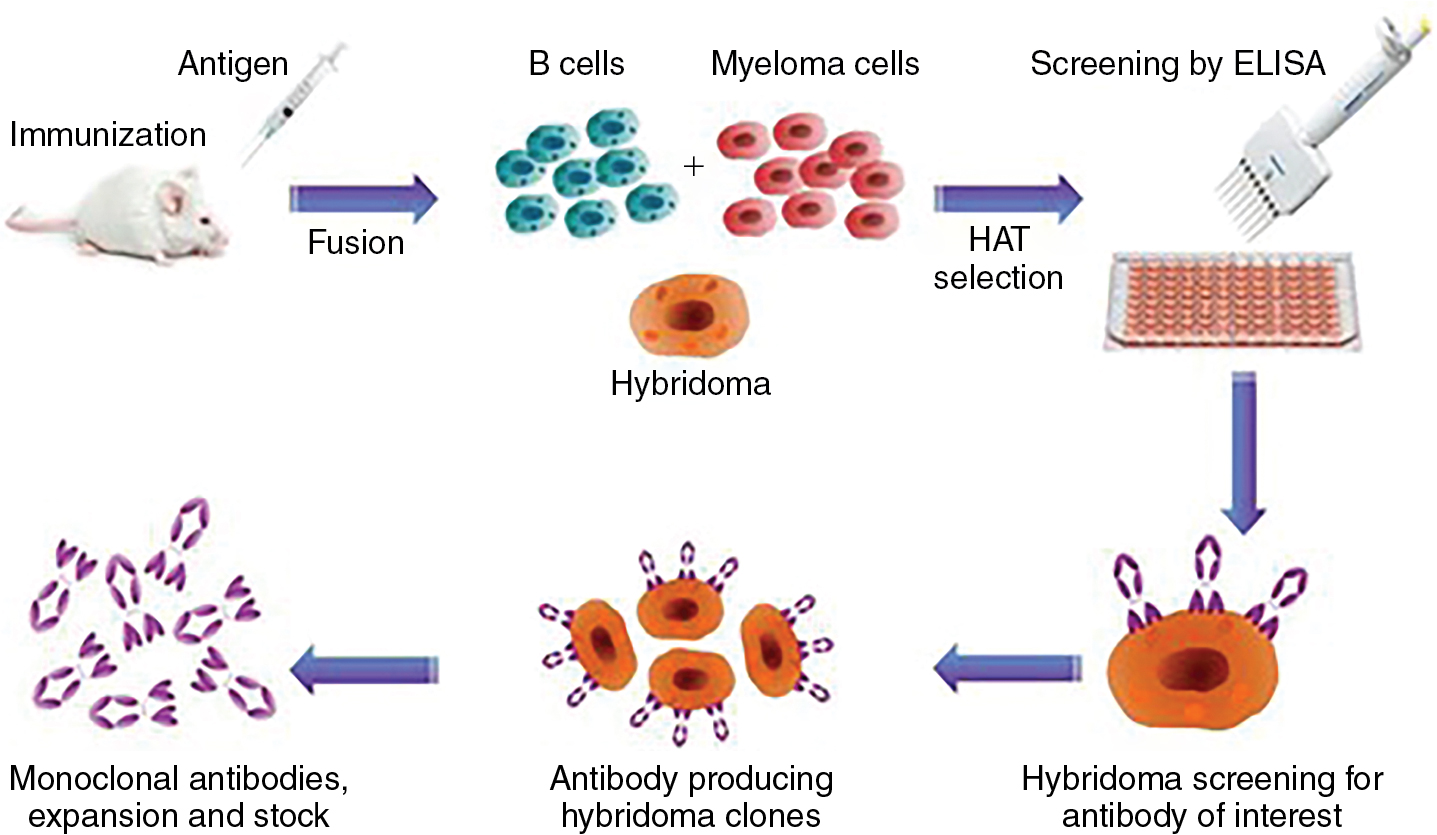
The development of hybridomas in the 1970s allowed for B-cell immortalization to expand rare clones. With this approach, mice could be immunized with tumor cells and tumor-specific targets could be identified ( Fig. 6.3 ). In the late 1980s and early 1990s, several investigators were able to isolate T cells reactive to mucin antigens from patients with epithelial cancers. A major advance came with the identification of the cancer testis antigens, with expression normally restricted to germ cells. Many tumors express these genes, particularly melanoma. Cancer testis antigens include MAGEA1, NY-ESO-1, SSX, and many others. MAGEA1 was first cloned in 1991 and found to be recognized by T cells in a human leukocyte antigen (HLA)-dependent manner.