Summary of key facts
- •
Immunoglobulins (Igs) are the soluble mediators of antigen recognition. An Ig that is capable of specifically binding an antigen is termed an antibody .
- •
Immunoglobulins range in molecular weight from 150 kD to 900 kD and are built from tetrameric units consisting of two heavy (H) chains and two light (L) chains, each chain containing one variable (V) domain and one or more constant (C) domains.
- •
There are five major classes of human Igs (IgM, IgD, IgG, IgA, and IgE), distinguished by their H chain constant regions.
- •
The Ig classes differ with respect to their effector functions. The antigen-binding sites of Ig are located in the V regions of the paired heavy and light chains.
- •
Antibody–antigen binding exhibits a range of affinities, from K A ≈ 10 M −1 to K A ≈ 10 M −1 .
- •
Ig genes are assembled from arrays of V, D, and J gene segments by V(D)J recombination.
- •
Constraints on V(D)J recombination enforce allelic exclusion, the process by which a given B cell expresses only one Ig.
- •
The affinity of Ig for antigen is increased during B-cell maturation by consecutive rounds of somatic hypermutation (SHM) and selection. SHM is initiated by the enzyme activation–induced deaminase (AID).
- •
A process related to SHM, class switch recombination (CSR), allows a B cell that expresses IgM and IgD of a defined antigenic specificity to express other Ig classes of the same specificity.
- •
CSR, like SHM, is initiated by AID.
- •
Defects in V(D)J recombination, SHM, or CSR are associated with primary immunodeficiency disorders.
- •
The Ig constant regions are subject to glycosylation and the resulting glycan modifications are structurally diverse.
- •
Glycosylation modulates the half-life, inflammatory activity, and cytotoxic effector function of Ig. Glycosylation also occurs at somatically mutated sites in Ig variable regions and can promote survival of some B-cell lymphomas.
- •
The effector functions of Ig include neutralization of antigen, opsonization, recruitment of complement, and recruitment of other immune cells.
- •
Of particular importance to immuno-oncology is antibody-dependent cellular cytotoxicity (ADCC), a process by which effector cells bearing Ig receptors recognize and lyse target cells coated by specific antibodies.
- •
The main ADCC effectors are natural killer (NK) cells.
- •
Monoclonal antibodies (mAbs), in distinction from the polyclonal antibodies characteristic of a natural immune response, possess a unique primary amino acid sequence and are directed against a single antigenic epitope.
- •
More than 100 mAbs have been approved for therapeutic use in the United States. They are derived by natural immunization and cellular cloning or by more direct methods of genetic engineering.
- •
Earlier generations of mAbs were generated in mice and deleterious immune responses in humans were mitigated by synthesis of mouse–human chimeras; more recently the generation of humanized mice and direct cloning from human B cells have obviated the immunogenic properties of mAbs derived from nonhuman sources.
The structure of immunoglobulins
Overall structure
Immunoglobulin (Ig) and T-cell receptors (TCRs), produced by B- and T-lymphoid cells, respectively, are the specific antigen recognition units of the adaptive immune system. Whereas TCRs are exclusively associated with the cell surface, Ig is present in both membrane-bound and secreted forms. This chapter will focus on Ig, although it should be noted that Ig and TCRs share a number of similarities, particularly with respect to the structure and genetic encoding of their antigen-binding sites.
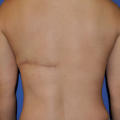
The term antibody denotes an Ig capable of recognizing a specific antigen. In soluble form, antibodies are the main effectors of B-cell-mediated adaptive immunity. Antibodies are large, multimeric proteins ranging in molecular weight from about 150 kD (IgG) to 900 kD (IgM). All classes of antibodies are built from one or more heterotetrameric, Y-shaped units; the basic unit is depicted in Fig. 2.1 for IgG. Each heterotetramer consists of two identical heavy (H) chains and two identical light (L) chains of molecular weight 50–75 kD and 23 kD, respectively. The H chains are joined covalently by disulfide linkages, as is each L chain to its neighboring H chain (see Fig. 2.1 ). ,
Historically, partial proteolysis was used to dissect the functional regions of Ig molecules. Some of these proteolytic fragments retain significance today as therapeutic agents. Digestion of IgG with papain cleaves each H chain at the amino-terminal side of the inter-H chain disulfide linkages, thereby releasing two antibody fragments, termed Fab, each containing a single antigen-binding site (see Fig. 2.1 ). Digestion of IgG with the protease pepsin cleaves each H chain at the carboxy-terminal side of the inter-H chain disulfide bonds (see Fig. 2.1 ). , This produces a fragment termed F(ab′) 2 , in which the two antigen-binding sites remain linked, and an Fc fragment, consisting of the paired carboxy-terminal C H domains. The Fc fragment is responsible for diverse effector functions, including antibody-dependent cytotoxicity (ADCC), opsonization, and complement fixation. ,
Structures of the Ig light and heavy chains
The synthesis of functional antibody light and heavy chains underlies the generation of humoral immune responses. The expression of Ig H and L chains is initiated in an ordered fashion during B-cell development, and this ordering is a consequence of the regulated assembly of Ig heavy chain (IgH) and Ig light chain (IgL) genes, as described in detail below.
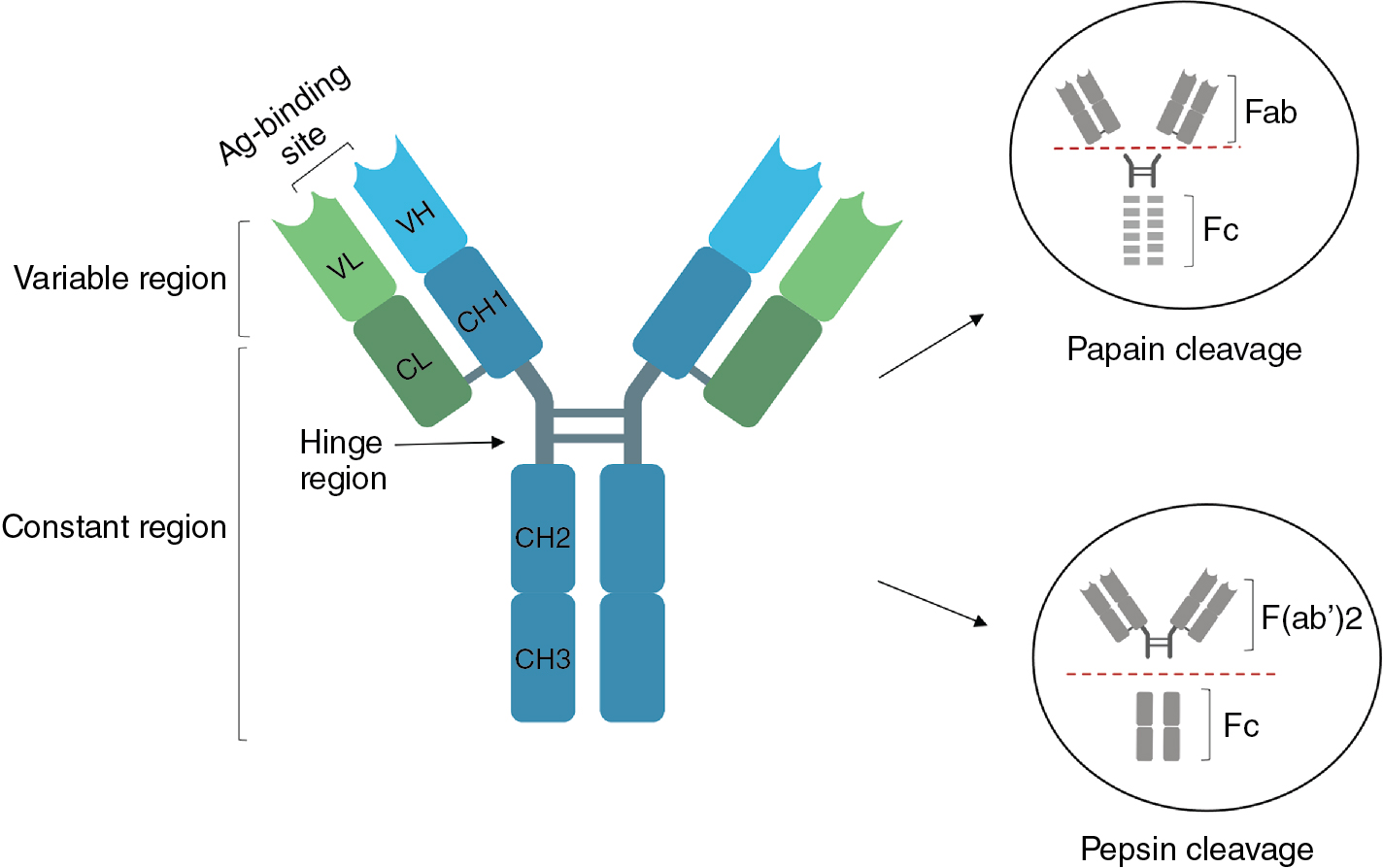
The Ig chains are built up from globular protein domains, each approximately 110 amino acid residues in length. These domains share a common structure called the immunoglobulin fold , consisting of two β sheets linked by a disulfide bond and surrounding a hydrophobic core. Each light chain comprises two immunoglobulin domains, termed V L and C L , for variable and constant, because the amino-terminal V L domain is variable in sequence and the carboxy-terminal C L domain is constant in sequence for a given class of light chain (see Fig. 2.1 ). , The antibody heavy chains contain four or five immunoglobulin domains, depending on the antibody class (see later): V H and C H 1 through C H 3 or C H 4, where V and C denote variable and constant, as for the light chains. In a folded, heterotetrameric Ig molecule, the V H and V L domains are closely associated and form the antigen-binding site; the C H 1 and C L domains are likewise associated, as are the paired C H 2, C H 3, and, when present, C H 4 domains ( Fig. 2.2 ). ,
Ig molecules are not rigid but contain flexible regions that allow the antibody to conform to the arrangement of binding sites in multivalent ligands. The first of these flexible regions, the elbow, is formed by the relatively unstructured polypeptide sequences that link V L and C L and V H to C H 1 (see Figs. 2.1 and 2.2 ). All antibody classes except for IgM and IgE contain a second flexible region, the hinge, which permits the Fab arms of the antibody to rotate and bend relative to the Fc. The hinge region also contains the interheavy chain disulfide bonds and differs in length among H chain classes, as will be discussed in the following section.
Antibody–antigen binding
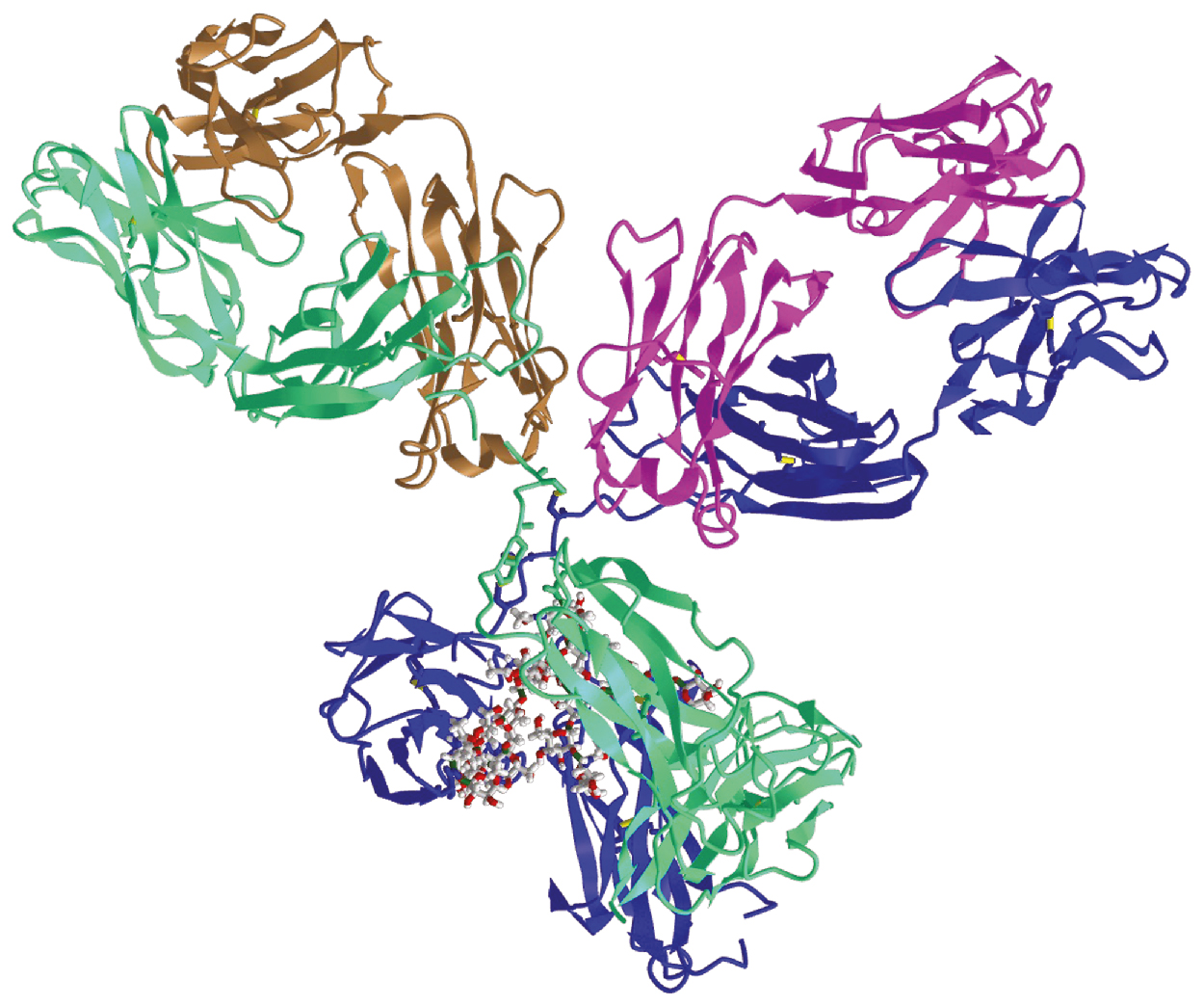
The V regions of Ig H and L chains are highly variable in sequence, but this variability is not evenly distributed along the lengths of the V H and V L regions. Rather, in each V region, sequence variability is clustered in three distinct locations called the complementarity-determining regions 1 through 3 (CDR1–CDR3). These hypervariable regions span residues 24 to 34, 50 to 56, and 89 to 97 in the human V L domains and residues 31 to 35, 50 to 65, and 95 to 102 in the V H domains. , , As a consequence of imprecision in the assembly of Ig genes, as described later, the lengths of the third CDRs are variable. In the folded, assembled tetraheteromeric antibody molecule, the three CDRs of each Ig chain are brought into proximity to form a single surface that constitutes the antigen-binding site ( Fig. 2.3 ). Because the amino acid residues that comprise the CDRs are highly variable in sequence, it follows that the surface that they form is highly variable in shape. Within a given Ig molecule, the two H chains are identical, as are the two L chains; therefore the two antigen-binding sites are identical.
The surfaces formed by the six CDRs are large, with typical areas ranging from 700 to 750 Å. In crystal structures of Fab fragments in complex with protein antigens the interacting antigenic surface, termed an epitope , is of a similar area. Binding between these two surfaces is mediated by hydrophobic interactions, van der Waals forces, hydrogen bonds, and electrostatic interactions. The binding of Ig to its cognate antigen is reversible; antibody–antigen affinities are measurable and can be expressed as association constants (K A ). For the binding of an antibody to protein antigens, a wide range of affinities is observed, from K A ≈ 10 M −1 for the lowest affinity interactions to K A ≈ 10 M −1 for very high-affinity binding. It is important to note that these affinities refer to the binding of an antigen to a single antigen-binding site. Because antibodies are multivalent, the binding of an antibody to a multivalent antigen can involve the simultaneous engagement of two or more antigen-binding sites. The strength of such an interaction is called avidity and can greatly exceed the affinity measured for monovalent binding. ,
Antibody isotypes
Characteristics of human antibody isotypes
In humans, there are five primary immunoglobulin classes: IgM, IgD, IgG, IgA, and IgE; each immunoglobulin class is defined by its heavy chain isotype: mu (μ), delta (δ), gamma (γ), alpha (α), and epsilon (ε), respectively ( Fig. 2.4 ). All immunoglobulin classes employ the same light chain isotypes, which are either kappa (κ) or lambda (λ). ,
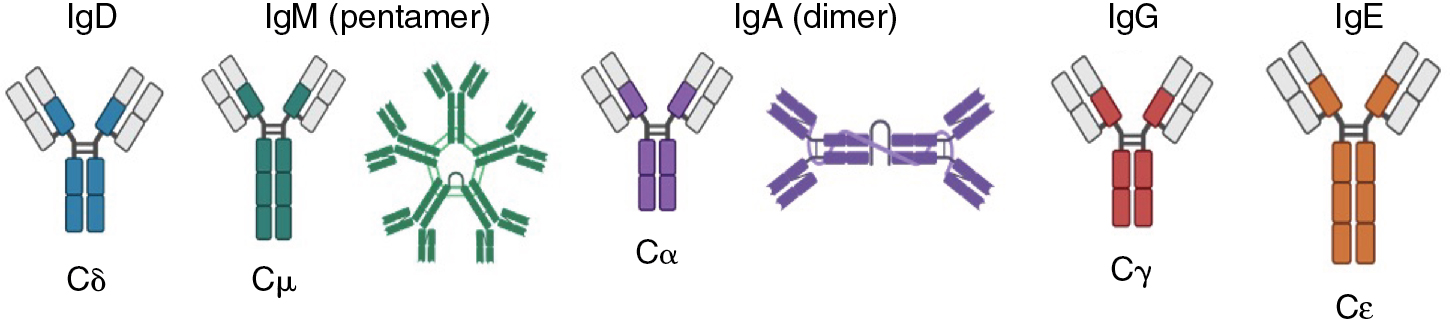
The IgG class contains four subclasses: IgG1, IgG2, IgG3, IgG4. The IgA class comprises two subclasses, IgA1 and IgA2. The IgG and IgA subclasses are differentiated by the structures of their hinge and tail regions, which confer distinct functional characteristics ( Table 2.1 ). ,
| Immunoglobulin | % in Serum (Adults) | Molecular Weight (kD) | Structure | Half-Life (Days) | Amino Acid Residues in Hinge Region | Placental Transfer | Opsonization | Complement Activation |
|---|---|---|---|---|---|---|---|---|
| IgG | 75 | +++ | ||||||
| IgG1 | 67 | 146 | H2L2 | 23 | 15 | ++++ | ++ | |
| IgG2 | 22 | 146 | H2L2 | 23 | 12 | ++ | + | |
| IgG3 | 7 | 165 | H2L2 | 7–21 | 62 | ++/++++ | +++ | |
| IgG4 | 4 | 146 | H2L2 | 23 | 12 | +++ | − | |
| IgA | 15 | − | ||||||
| IgA1 | (H2L2)2 | 5.5 | 22 | − | − | |||
| IgA2 | (H2L2)2 | 5.5 | 9 | − | − | |||
| IgM | 10 | 970 | (H2L2)5 | 5–10 | − | − | + | +++ |
| IgE | <0.01 | 188 | H2L2 | 2 | − | − | − | − |
| IgD | <0.5 | H2L2 | 2.8 | 58 | − | − | − |
In addition to their secreted forms, all Ig classes have corresponding membrane-bound forms in which the heavy chain isotypes differ from those of the secreted forms at the carboxyl terminus: the membrane-bound heavy chains have an extended peptide sequence containing a negatively charged extracellular juxtamembrane domain, a transmembrane domain, and a positively charged anchor sequence. Membrane-bound Ig associates with the Igα/Igβ heterodimer (CD79) to form the B-cell receptor (BCR) complex, which transduces antigenic signals. Mature naïve B-cells and unswitched memory B-cells express two membrane-bound Ig classes, IgD and IgM. Further maturation of B cells is accompanied by class switching, a process by which IgD and IgM are replaced by IgG, IgA, or IgE at the cell surface (see Class switch recombination and somatic hypermutation ). Ig-bearing memory B cells reside in lymphoid tissues and peripheral blood, where they comprise approximately 21% to 28% of the circulating pool of B cells in adults. Notably, IgA+, IgG+, and IgM+IgD+ B cells are differentially distributed across tissues. For example, human tonsils contain a larger frequency of IgA+ B cells than other organs, IgM+IgD+-expressing naïve B cells are the predominant isotype in peripheral blood, lymph nodes, and the bone marrow, and IgG+ cells seem to be evenly distributed across most tissues. In contrast, IgE+ B cells appear only transiently and in low numbers in germinal centers.
Soluble antibodies are produced by terminally differentiated B cells collectively known as antibody-secreting cells (ASCs), which arise from activated B cells. ASCs include short-lived effector cells that arise early in antibody responses (plasmablasts and short-lived plasma cells) and long-lived plasma cells that are responsible for durable antibody responses. Short-lived ASCs can arise in response to T cell–independent antigens or during the early responses to T cell–dependent antigens. Conversely, the generation of long-lived ASCs requires cell-to-cell interactions with T cells in specialized microenvironments known as germinal centers (GCs). GCs are located in the follicles of lymphoid organs and promote the development of high-affinity memory B cells and long-lasting plasma cells, responsible for long-term antibody production. Long-lived ASCs reside in gut-associated lymphoid tissues (GALT) and in the bone marrow, where microenvironmental signals are required for their longevity. , Long-lived ASCs predominantly produce IgA and IgG. In contrast, IgE+ ASCs are rarely found, perhaps because most IgE+ B cells differentiate into short-lived ASCs.
Biologic functions of Ig isotypes
IgM. IgM is the first immunoglobulin produced during B cell development and its membrane-bound form is initially found on the surface of naïve B cells. In response to T cell–independent antigens and early during the response to T cell–dependent antigens, B cells differentiate into plasmablasts and short-lived plasma cells, which secrete soluble IgM. IgM is usually secreted as a pentamer ((H2L2)5), comprising IgM monomers (H2L2) that are covalently linked by disulfide bonds. The IgM pentamer also contains a 137 amino acid–long polypeptide termed J-chain that facilitates IgM multimerization and secretion at mucosal surfaces. , The pentameric structure of soluble IgM confers high avidity for polyvalent antigens because each IgM molecule contains up to 10 functional antigen-binding sites. The relatively high avidity of such polyvalent interactions can compensate for the relatively low affinity of IgM, which is generated during primary antibody responses before the onset of affinity maturation. Importantly, IgM efficiently supports complement fixation and opsonization of antigen, which are its two main effector functions. Additionally, natural antibodies with innate-like properties are of the IgM class. Specifically, natural IgM is present from birth without external antibody exposure and acts as the first line of defense against invading pathogens. Natural IgM also has an important role in the recognition and clearance of apoptotic cells and regulation of inflammation and self-tolerance, contributing to tissue homeostasis.
IgD. In its membrane form, IgD is expressed on the surface of late transitional and mature naïve B cells, where it is often co-expressed with IgM (IgD+IgM+ B cells). Co-expression of IgD and IgM is regulated through alternative splicing, and the usage of Cμ and Cδ varies widely across B cells. In contrast to IgM, the expression of IgD is transient, and IgD disappears from the cell surface upon B cell activation. Because IgD and IgM co-expression occurs only during a narrow developmental window in B cells, membrane-bound IgD is thought to play an important regulatory role in the fate of naïve B cells. Furthermore, observations that IgD BCRs sense endogenous antigens less efficiently than IgM BCRs suggest that the balance of IgM and IgD co-expression on subsets of mature B cells (e.g., naïve follicular B cells) may contribute to the development of self-tolerance. ,
In humans, a subset of B cells expressing IgD only (IgM−IgD+) is found in the respiratory mucosa and its associated lymphoid organs (e.g., tonsils, cervical lymph nodes) and glands (e.g., salivary and lacrimal). , IgM−IgD+ B cells originate through a noncanonical form of class switch recombination and, characteristically, use only λ light chains. In addition, the mucosal microbiota regulate IgM-to-IgD CSRs in response to airborne and oral antigens, suggesting an important role of IgM−IgD+ B-lymphoid cells in shaping the antigenic composition of the respiratory tract and their response to pathogens. ,
In its soluble form, IgD is mainly released by IgM−IgD+ plasma cells and is found in most extravascular body fluids (lacrimal, mammary, pancreatic, cerebrospinal, nasal, and bronchial fluids) except for the intestinal mucosa, where only traces of IgD are detected. Although lacking a specific Fc receptor, IgD binds mucosal basophils, mast cells, and to a lesser extent phagocytes, triggering the release of antimicrobial peptides. Secreted IgD may also suppress IgE-mediated degranulation of basophils and mast cells in response to allergens. IgD is also found in very low concentrations in serum, where it accounts for less than 0.25% of all immunoglobulin (see Table 2.1 ). ,
IgA. IgA, the most abundant Ig isotype in humans, is predominantly secreted at mucosal surfaces. IgA is also present in serum, comprising about 15% of circulating Ig. Serum IgA is monomeric (H2L2), whereas secretory IgA (sIgA) at mucosal membranes and in bodily fluids is usually dimeric ((H2L2)2) where the two IgA monomers are associated with J-chain by means of disulfide bonds. There are two subclasses of IgA that are largely differentiated by their hinge regions. IgA1 has a hinge region of 22 amino acid residues with linked glycans at serine and threonine. The long hinge region of IgA1 is susceptible to degradation by bacterial proteases. In contrast, IgA2 has a hinge region of nine amino acid residues that, although lacking linked glycans, is more stable. Hence IgA2 is the predominant subclass in mucosal surfaces that are rich in bacterial proteases, such as the colonic mucosa. ,
Human B cells may undergo class switching to IgA in the course of T cell–dependent or T cell–independent responses, giving rise to IgA antibodies of high or low affinity, respectively. T cell–dependent class switching is dependent on transforming growth factor beta (TGF-β) and binding of CD40 on the B cell to CD40L on the surface of the T cell. Conversely, T cell–independent class switching involves innate signals from Toll-like receptors and the dendritic cell (DC)-derived factors BAFF and APRIL, which promote B-cell activation and survival. Intestinal epithelial cells produce APRIL upon sensing bacteria through TLRs. APRIL triggers sequential class switching from IgA1 to IgA2 in B cells of Peyer patches, thereby enriching the production of IgA2 in the distal intestinal tract.
Secretory IgA (sIgA) is the main Ig class transferred to infants through breast milk. Indeed, the concentration of IgA reaches 12 mg/mL in antibody-enriched colostrum (up to 50% of the protein conferred to newborns) and averages about 1 mg/mL in mature milk. IgA in breast milk is produced by IgA plasma cells primed in maternal mucosal-associated lymphoid tissues that migrate to the lactating breast. Antibodies are transferred to breast milk through the epithelium of lactiferous ducts and alveoli. This epithelium expresses the polymeric Ig receptor (pIgR), which binds to the J-chain of polymeric IgA to form complexes that undergo endocytosis. Next, IgA is transported in vesicles to the apical epithelial side where the intramembranous domain of pIgR is cleaved, releasing IgA bound to an external fragment of the pIgR called the secretory component. The secretory component protects secreted antibodies from degradation by proteases at mucosal surfaces. In most mammalian species, antibodies from breast milk are absorbed in the distal gut and transported into the neonatal circulation by Fc-mediated transcytosis in enterocytes. In contrast, in human infants, breast milk antibodies do not enter the circulation and are primarily produced for host defense at mucosal surfaces. Because of its origin in plasma cells primed in maternal mucosa, the IgA transferred in human breast milk is directed primarily against commensal, gut, and respiratory antigens, providing neonates with protection against mucosal antigens found in the mother. Breast milk antibodies also play a critical role in establishing the infant’s commensal flora and immune responses during early life. ,
IgG. In humans, IgG is the most abundant Ig in serum and nonmucosal tissues, and it has the longest serum half-life of all immunoglobulin isotypes. IgG antibodies comprise four subclasses: IgG1, IgG2, IgG3, and IgG4. The four subclasses are similar, differing mainly in surface-exposed residues on the constant domains and the structure of their hinge regions. These differences confer specific properties on each subclass, including antigen-binding, half-life, complement activation, and placental transfer (see Table 2.1 ).
Consequently, IgG subclasses exhibit functional differences. Effector mechanisms include direct neutralization of pathogens and toxins, activation of complement-dependent cytotoxicity (CDC), antigen-dependent cellular phagocytosis (ADCP), and antibody-dependent cell-mediated cytotoxicity (ADCC). IgG-mediated complement activation is initiated by the binding of complement component C1q to the Fc region (CH2 domain), leading to pathogen opsonization by C3b and the formation of the membrane attack complex (C5–C9). IgG Fc domains can also activate complement by interacting with mannose-binding lectin. IgG1 and IgG3 are potent complement activators, whereas IgG2 and IgG4 are less effective. The characteristics of the Fc region also determine the affinity of each subclass for specific Fc receptors (FcγRs), thereby contributing to the regulation of downstream IgG effector mechanisms. There are six types of human FcγRs: I, IIa, IIb, IIc, IIIa, and IIIb. As a class, FcγRs belong to the Ig receptor superfamily and are localized on antigen-presenting and innate immune cells. IgG1 and IgG3 bind to all FcγR subclasses; IgG4 binds most strongly to FcγRI but also binds IIa, IIb, IIc, and IIIa with lower affinity, and IgG2 binds predominantly to FcγRIIa. ,
A distinct IgG Fc receptor, the neonatal Fc receptor (FcRn), is structurally similar to class I major histocompatibility complex (MHC). It is found on hematopoietic cells (namely, myeloid-derived innate immune cells, professional antigen-presenting cells, and vascular endothelia). FcRn also resides in hepatocytes and some epithelial cells, including podocytes, choroid plexus, and intestinal and respiratory epithelial cells. FcRn binds to IgG with high affinity under acidic conditions (pH < 6.5) but not at physiologic pH. This property plays a key role in IgG transport by defining locations in which IgG is bound or released. Prenatally, FcRn transcytoses IgG from the mother to the fetus across the placental syncytiotrophoblast. Throughout life, FcRn transports IgG at epithelial barriers via Fc-mediated transcytosis. Importantly, in APCs, FcRn governs the intracellular trafficking of IgG and IgG immune complexes into compartments where they are processed for antigen presentation. FcRn also protects IgG from degradation and recycles antibodies in endothelial, hematopoietic, and epithelial tissues, prolonging IgG half-life. , ,
IgE. IgE mediates immediate hypersensitivity reactions. IgE also participates in protective immune responses against helminths as well as viruses and toxins. IgE is extremely potent and therefore is the most tightly regulated of all Ig isotypes. Class switching to IgE is primarily T cell dependent, requiring interactions with T-follicular helper cells through CD40 and CD40L in addition to soluble signals such as interleukin (IL)-4 and IL-13. Alternatively, IgE can be produced independently by T cells in response to glucocorticoids, the B-cell survival factors BAFF and APRIL, complement C4b-binding protein, or IL-4 in the context of infection by Epstein-Barr virus (EBV). Conversely, class switching to IgE is inhibited by some cytokines (e.g., IL-10, IL-21, TGF-β) and cell surface receptors (e.g., BCR, CD45, CTLA4, FcεRII). ,
IgE+ B cells and IgE-secreting cells are short-lived and are rarely found in the peripheral blood or tissues of healthy individuals but increase under pathologic conditions associated with IgE overproduction, such as allergies, and are positively correlated with plasma IgE levels. , Soluble IgE, which has the shortest half-life of the circulating immunoglobulins (see Table 2.1 ), is typically present in serum at concentrations lower than 0.01% of serum Ig. IgE upregulates the expression of its receptors on target cells, which is one of the reasons for its potency. IgE binds with high affinity to FcεRI on the surfaces of mast cells, basophils, Langerhans cells, and eosinophils, stimulating degranulation and the release of inflammatory mediators. A low-affinity receptor for IgE, FcεRII or CD23, is constitutively expressed on B cells, where it promotes cell growth and negatively regulates IgE synthesis. An inducible form of CD23 is expressed on various hematopoietic cells and on epithelial cells, where it transports antigens from the cell surface to the basolateral membrane.
The genetic basis of immunoglobulin diversity
Organization and assembly of immunoglobulin loci
In humans, there are three Ig loci: the heavy chain locus (IGH) on chromosome 14q32, the kappa locus (IGK) on chromosome 2p11, and the lambda locus (IGL) on chromosome 22q11. At each locus, the corresponding Ig variable domain is encoded by an array of gene segments that are brought together during B cell development by a process termed V(D)J recombination. The IGH locus spans approximately 1.3 Mb. The heavy chain variable domains are encoded by three banks of gene segments, termed variable (VH), diversity (D), and joining (JH). At the 5′ end of the IGH locus, as defined by transcriptional orientation, there are about 50 functional VH segments, followed by 27 D segments and 6 JH segments ( Fig. 2.5 ). At the pro-B stage of B cell development, a D segment is joined to a JH segment; subsequently, a VH segment is joined to the DJH unit, thereby forming an intact variable coding sequence. To the 3′ side of the JH cluster are arrayed the exons encoding each of the heavy chain isotypes: μ, δ, γ3, γ1, α1, γ2, γ4, ε, and α2.
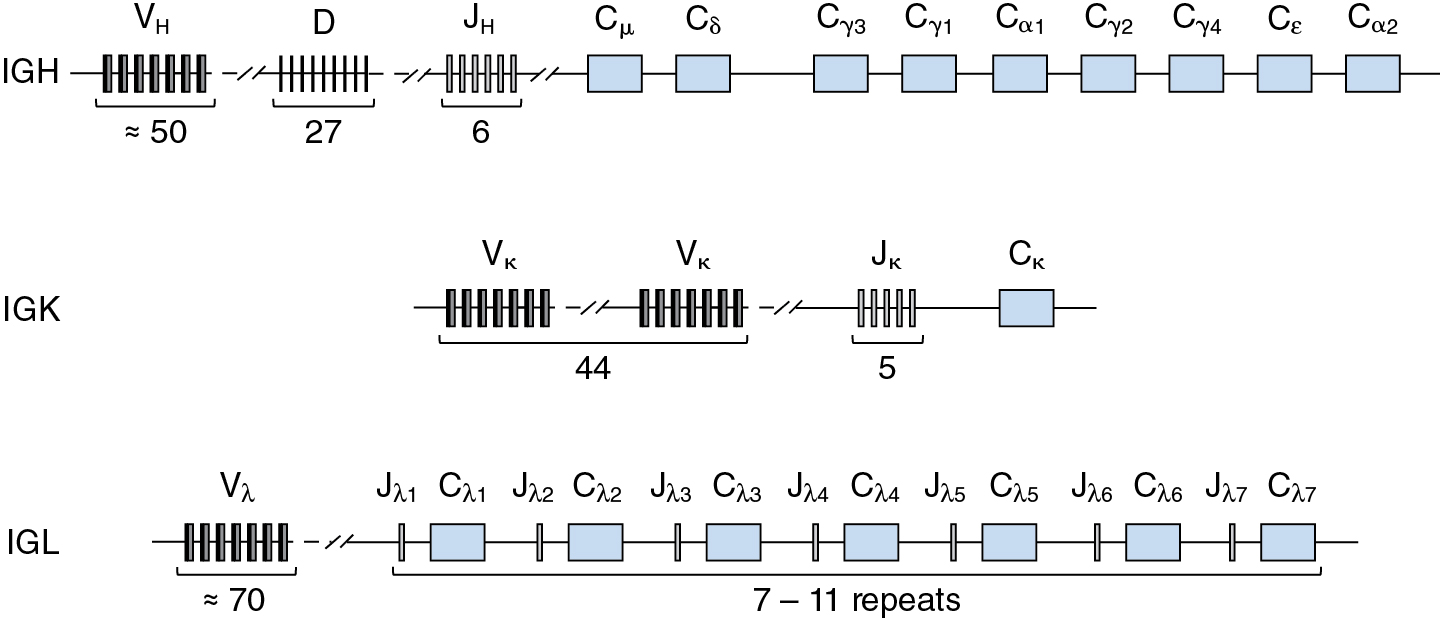
Whereas the heavy chain variable regions are encoded in three discrete DNA segments, VH, D, and JH, the variable regions of the kappa and lambda light chains are encoded in two DNA segments, VL and JL. The IGK locus, which spans about 1.7 Mb, contains an array of 44 functional Vκ segments, followed by a cluster of 5 Jκ segments and a single Cκ exon encoding the constant domain (see Fig. 2.5 ). The organization of the human IGL locus, which spans about 1.1 Mb, is more complex than that of IGK, consisting of about 70 Vλ segments and a cluster of from 7 to 11 Cλ exons, each flanked at the 5′ end by a Jλ segment (see Fig. 2.5 ). Despite the difference in organizational complexity, kappa and lambda light chain genes are assembled by a single recombination event that joins one VL segment to one JL segment.
During B cell development, activation of rearrangement at the light chain loci follows productive rearrangement at the heavy chain locus, which occurs at the pre–B cell stage. Rearrangement of the kappa locus generally precedes that of lambda.
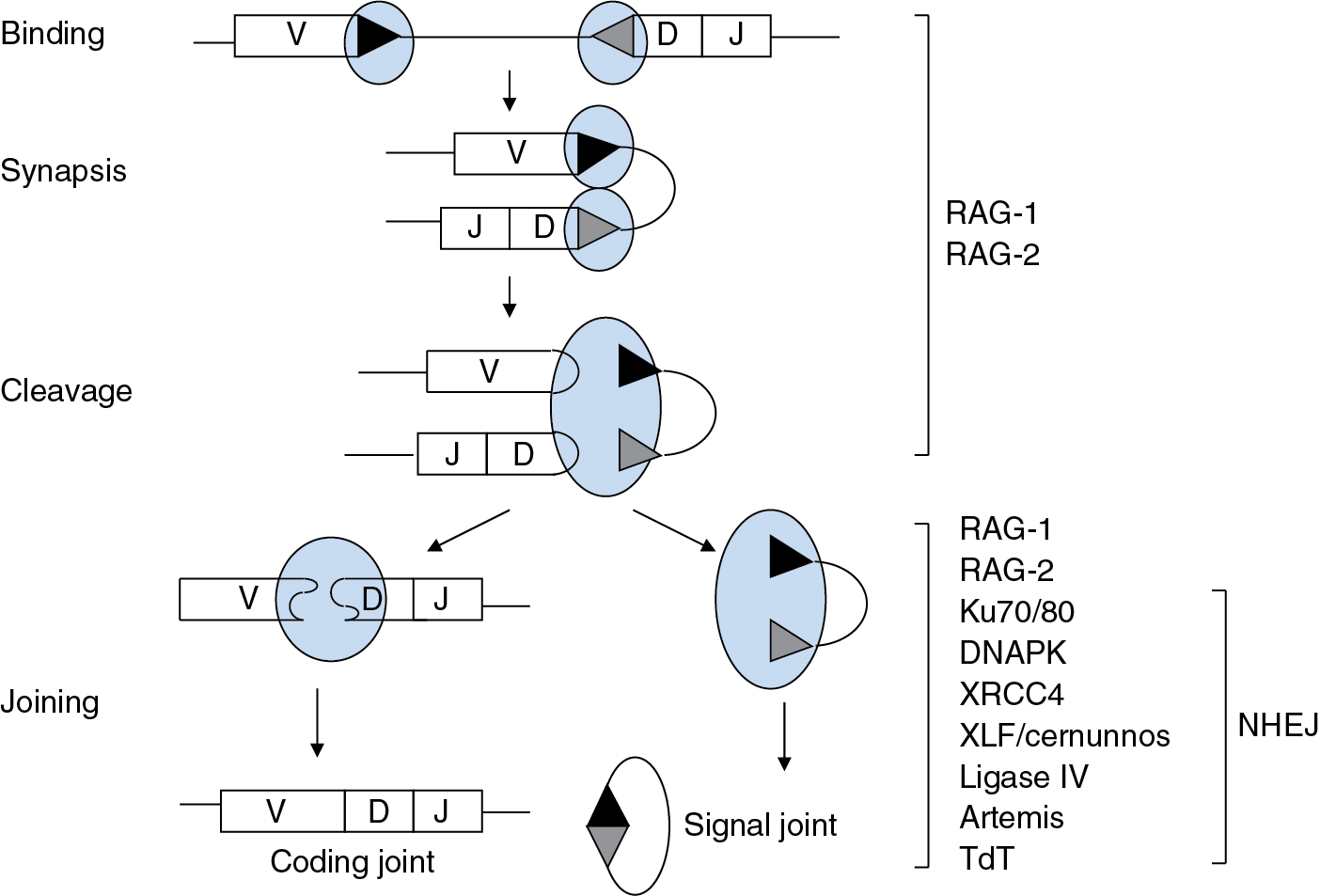
Mechanism of V(D)J recombination
V(D)J recombination is mechanistically related to bacterial DNA transposition and retroviral integration. Recombination is initiated by a specialized transposase, termed RAG (for recombination activating gene protein), consisting of two RAG-1 subunits and two RAG-2 subunits. Each V, D, or J gene segment is tagged with a recombination signal sequence (RSS) consisting of conserved heptamer and nonamer elements, separated by a less highly conserved spacer of 12 or 23 bp. These RSSs are the functional analogs of transposase binding sites in bacterial transposition and long terminal repeats in retroviral integration. The RAG tetramer brings together a pair of participating gene segments by binding to their RSSs; this process is constrained by the geometry of RAG so that synapsis can only occur between RSSs of differing spacer lengths. Thus the RSSs not only represent binding sites for the RAG complex but also play an essential role in determining which segments can join to each other. Upon synapsis, RAG nicks each gene segment at the junction between a recombination signal sequence and the coding portion of the gene segment. Nicking is followed by a transesterification reaction that produces a coding end, terminating in a hairpin, and a 5′-phosphorylated, blunt signal end ( Fig. 2.6 ).