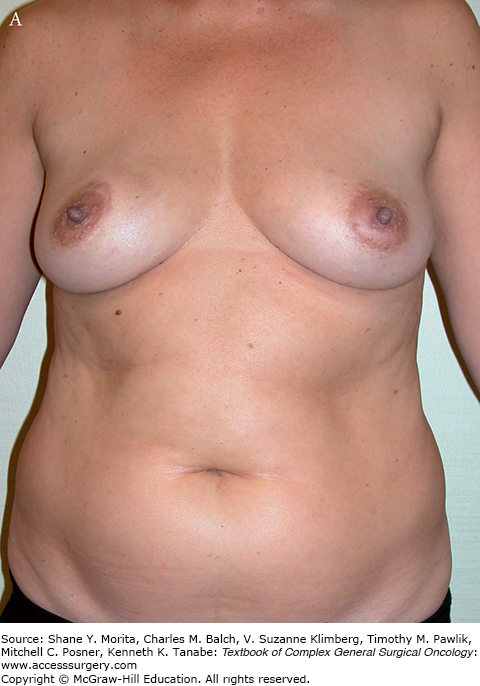
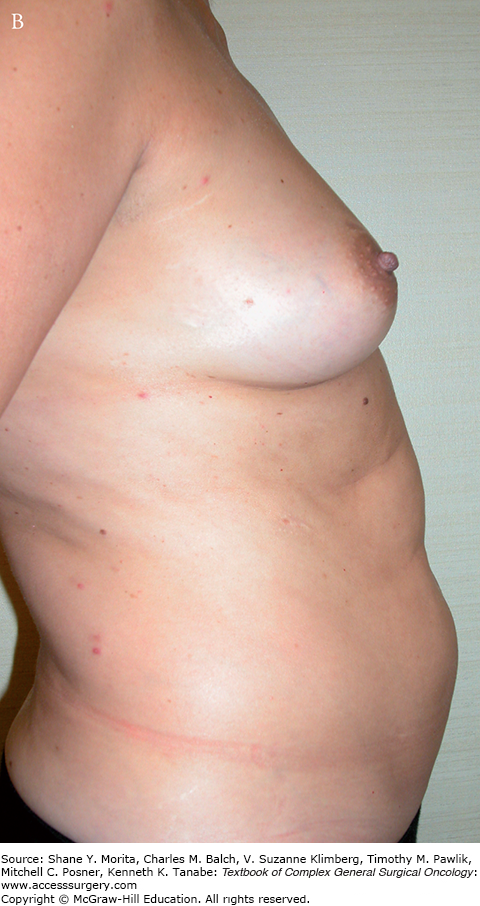
Breast reconstruction is an important aesthetic and rehabilitative option that should be offered and considered by every woman diagnosed with breast cancer. Breast reconstruction is not just for patients who are going to have a mastectomy, but may also apply to patients undergoing a lumpectomy or partial mastectomy. The number of women choosing to have breast reconstruction continues to increase on a yearly basis.1 Numerous studies have shown improved psychological well-being associated with breast reconstruction in the recovery period of women undergoing breast cancer treatment.2,3 Along with low patient morbidity, breast reconstruction has not been shown to have any deleterious effects in the overall oncologic treatment outcome.4–6 “Autologous” tissue reconstruction restores the appearance of the breast after mastectomy with the patient’s own tissue. The goal of reconstructive surgery is to recreate the most natural appearance, shape, and feel of a breast. The options for breast reconstruction have dramatically increased over the past 30 years with technical advances in surgery, and physicians can now offer cosmetically acceptable breast reconstruction by using tissue from numerous areas of the body.
Microsurgery involves the use of the operating room microscope to repair blood vessels that are otherwise too small to manipulate surgically with the naked eye. Today, surgeons can transfer units of tissue from one part of the body to another to repair various physical defects and deformities. Large tissue units or “flaps” need to be perfused with blood in order to survive, such that a unit of tissue can be transferred with its associated artery and vein to a separate region of the body where the blood vessels can be reconnected (anastomosis) to local recipient blood vessels. In terms of autologous tissue breast reconstruction, physicians can utilize microsurgery to transfer skin and fat as needed to make an entire breast.
In this chapter, we discuss the clinical considerations when evaluating patients for autologous flap breast reconstruction. Please note that in practice, patients are comprehensively evaluated for their candidacy for breast reconstruction and that recommendations should be individualized such that patients consider not only autologous tissue, but also expander implant–based reconstruction. Here we limit our discussion to describing the main surgical autologous tissue flap procedures along with their indications. We hope that by the end of the chapter the reader and health care professional will have a working knowledge of the vast reconstructive armamentarium available today for breast reconstruction to be able to better counsel patients.
Determining whether an implant-based or autologous reconstruction will be performed depends considerably on the patient’s body habitus and desires. Many women prefer to avoid the use of implants, which are essentially mechanical devices with a limited lifespan. Instead, patients may seek to enjoy the collateral benefit of the resultant flap harvest donor area, which may produce results that are similar to cosmetic procedures, such as removal of excess subcutaneous tissue when an abdominal flap is used resulting in a “tummy tuck.” However, unlike cosmetic procedures, autologous tissue flaps must be harvested on a specific blood supply in order to maintain their perfusion.
The different donor sites available, which will be discussed subsequently in this chapter, must be explained to the patient along with the expected deficits and scars. Additional surgeries are common following the initial reconstruction. These can include nipple and areola reconstruction, liposuction for contour refinements, scar revisions, fat injections, and flap reshaping. Fully informed patients will always be more satisfied. Counseling can be challenging since the patient may still be dealing psychologically and emotionally with the recent diagnosis of breast cancer.
Physicians and patients must keep in the mind that oncologic treatment of breast cancer should not be compromised by concerns for breast reconstruction. Regardless of the anticipated defect size or location, breast oncologic surgeons must be allowed to perform the appropriate cancer operation, even if it means making larger incisions and scars. Plastic surgeons and breast oncologists should work together in a collaborative fashion with the patient to determine the best treatment that satisfies everyone’s goals. This requires communicating preoperatively so that appropriate expectations are set. In addition, the effect of postoperative chemotherapy and radiation will need to be addressed. Patients should know that infections or delayed wound healing related to surgery could delay adjuvant therapies. Overall, for the majority of patients, breast reconstruction is a very safe and appropriate procedure.7,8 When evaluating patients for breast reconstruction, it is best to be involved with a multidisciplinary team early in the patient care process. Coordination with oncologic surgeons, medical oncologist, and radiation oncologist allows for breast reconstruction in the most optimal fashion.
“Immediate” breast reconstruction is reconstructive breast surgery performed at the same time as the mastectomy in one operative setting. It may seem obvious that the majority of women would prefer to have an immediate breast reconstruction. This allows the patient to have a breast immediately, and avoids the psychological discomfort of living with a mastectomy defect. Simultaneous reconstruction also allows for one less surgery and can provide for preservation of the breast skin “envelope.” However, there are circumstances in which a delayed breast reconstruction may be preferred and recommended.
“Delayed” breast reconstruction is performed after a mastectomy has already been completed. This may be weeks, months, or years later. Since not every facility is staffed with a plastic surgeon, a patient may undergo a mastectomy and then have her breast reconstruction performed elsewhere. However, even when immediate breast reconstruction is technically feasible, it may not be advised. As described below, radiation therapy is one of the most important factors when determining the type and timing of breast reconstruction. The majority of plastic surgeons believe that postmastectomy radiation therapy can adversely affect any reconstructive surgical option and hence prefer to perform breast reconstruction after all oncologic therapy is completed. Patients who have autologous tissue reconstruction and then have radiation therapy have increased risk of fibrosis and potential loss of volume. Furthermore, radiation therapy changes tissue characteristics permanently such that future surgeries to revise radiated tissue is prone to prolonged edema, scarring, fibrosis, delayed healing, and overall suboptimal aesthetic result. Therefore, in patients with a history of radiation, many plastic surgeons prefer to avoid implants and perform autologous tissue flap reconstruction. Once all adjuvant therapy has been completed, breast reconstruction can be considered.
Physician familiarity of the different flap options and proficiency in microvascular techniques are needed to successfully perform autologous breast reconstruction. Though there are numerous benefits to autologous over implant-based reconstructions, there are certain aspects that need consideration before proceeding. All patients who are evaluated for an extended operation and deemed “healthy” are potential candidates for autologous breast reconstruction. Autologous reconstruction is a significantly longer operation than implant-based reconstruction, and may take 4 to 6 and 8 to 10 hours to perform for unilateral and bilateral reconstructions, respectively. Systemic risk factors such as cardiac disease, pulmonary disease, renal disease, and other chronic illnesses that preclude prolonged anesthesia would not be appropriate to proceed with free flap reconstruction. Other risk factors such as smoking, diabetes, and obesity can increase the wound healing complications of the donor site and breast but are not absolute contraindications.9 A body mass index greater than 30 kg/m2 has been associated with increased wound-healing complications, particularly in the abdominal donor site area.9,10 And of course, patients must have enough donor tissue volume to adequately reconstruct the appropriate breast size. This can be assessed by a “pinch” test in which the proposed skin and fat are “pinched” to estimate the amount of volume.
Indications for the specific free flap used for reconstruction depend upon multiple factors including the patient’s body habitus, and experience and preference of the surgeon. A comprehensive preoperative assessment of native breast size, breast volume, body habitus, and donor site tissue volume help guide the decision-making process. Though patients may have contraindications to certain free-flap reconstruction, such as a previous abdominoplasty that eliminates the use of the abdominal-based flaps, other options may still remain. It is unlikely that a patient would have absolutely no free-flap options, but if this were the case, implant-based reconstruction may still be an option. Given the concern postoperatively for microvascular thrombosis, hematological disorders associated with abnormal clotting are not advised to undergo free-flap reconstruction, such as Factor V Leiden, lupus anticoagulation, protein C/S deficiencies, and other hypercoagulable states. Thrombosis of the blood vessels used to transfer autologous tissues would lead to ischemia and eventually necrosis of the tissues—that is, failure of the reconstruction. In some circumstances, patients with a poor oncologic prognosis or who have major comorbid conditions and significant systemic illnesses may be better served with the less invasive option of an implant reconstruction.
As with any surgery, thorough preoperative counseling of the expected reconstructive result is important. Though technical advancements have allowed for excellent aesthetic results in breast reconstruction, there remain limitations.
Improved knowledge of abdominal wall anatomy and blood supply has allowed for the development of abdominal wall–based flaps for breast reconstruction. The paired rectus abdominis muscles run in parallel on either side of the abdominal midline. This muscle has two main blood supplies or dominant “vascular pedicles”: superiorly from the superior epigastric artery/vein and inferiorly from the deep inferior epigastric artery/vein (DIEA/V).
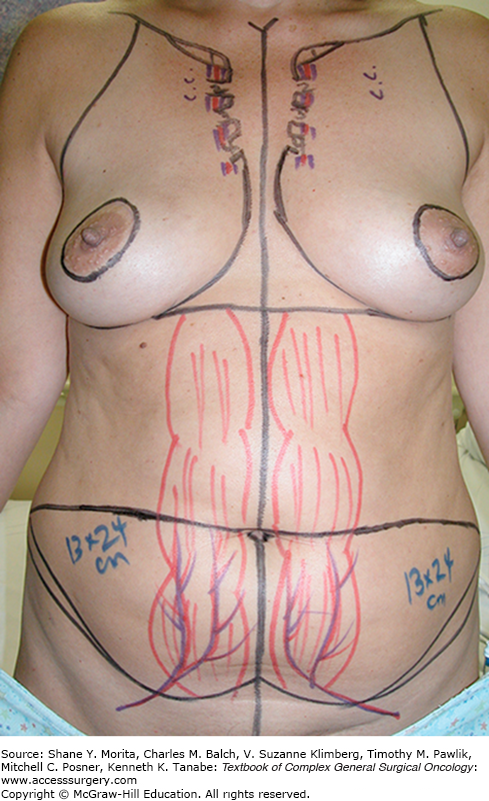
With anatomical studies elaborating on the blood supply to this area, the lower abdomen has become the most common donor site. The flaps utilize the transverse portion of lower abdominal wall skin and soft tissue. In many patients there is sufficient volume of skin and fat to reconstruct one or both breasts. The flaps are raised above the abdominal muscular fascia from lateral to medial, and include the skin and full-thickness subcutaneous fat tissues. Once the border of the rectus muscle is reached, further dissection depends on the vascular anatomy and type of flap being harvested. Unilateral or bilateral reconstruction can be performed since the blood supply for each half of the abdomen is separate and distinct.
The transverse rectus abdominis myocutaneous (TRAM) flap was originally described as a pedicled flap based upon the superior epigastric vessels.11 The pedicled TRAM flap typically requires dissection of the entire rectus muscle off of its attachments to the pubic bone. Modifications in this technique are attempts to leave some of the rectus muscle in place; however, extensive dissection of the muscle is still required in a pedicled TRAM flap.
The main drawback is the sacrifice of the entire rectus muscle. The loss of the rectus muscle from the abdomen leads to abdominal wall weakness that can affect a woman’s ability to perform strenuous physical activity, including exercise. In addition, the superior epigastric vessels contribute to a lesser extent to the blood supply of the lower abdomen; hence, the pedicled TRAM flap has a higher rate of partial flap necrosis and fat necrosis. It is the authors’ preference that a pedicled TRAM flap rarely be performed and only when microsurgery is not an option.
With advancements in microsurgical techniques, the free TRAM flap has become more commonly performed.12 The DIEAs/Vs provide a greater blood supply to the lower abdominal skin and fat, and consequently, there are less problems with partial flap necrosis and fat necrosis. The DIEAs/Vs are divided, the entire tissue is moved to the chest, and then the blood vessels are reconnected to local recipient blood vessels. As with the pedicled TRAM flap, the entire rectus muscle is harvested along with the overlying lower abdominal skin and subcutaneous tissues. With this technique, there is still the risk of developing postoperative hernias and bulges.
As a natural evolution in operative technique, surgeons sought to minimize sacrifice of the rectus abdominis muscle, but still are able to transfer the same amount of abdominal tissue. The DIEA/V commonly divides into medial and lateral branches before entering the rectus muscle. These branches give rise to a medial and lateral row of perforating blood vessels (aka “perforators”) that then supply the overlying skin and fat. Therefore, it is technically possible to harvest only a small cuff of rectus muscle that surrounds the chosen perforating vessels in order to harvest the lower abdominal flap. The benefit is that it spares a portion of the rectus abdominis muscle and, therefore, is meant to preserve as much abdominal wall strength and minimize the risk of hernia or bulging.13
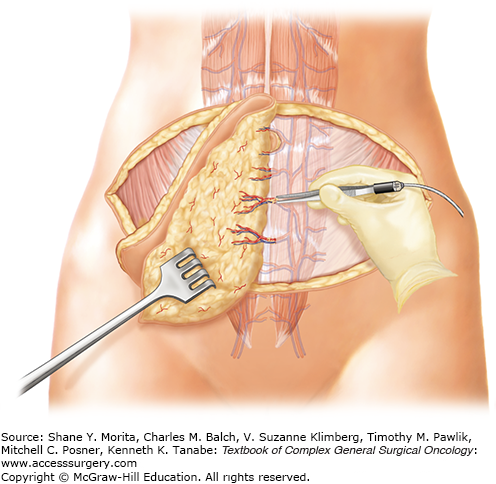
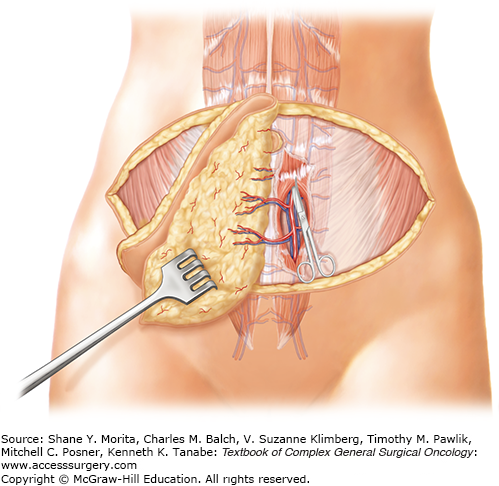
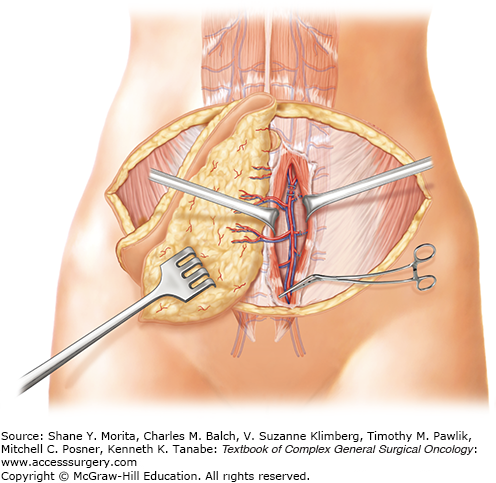
Taken one step further, surgeons sought to leave the entire rectus abdominis muscle in the abdomen and harvest only the skin, fat, and perforating blood vessels.14 The same skin area is excised and utilized for the breast reconstruction as previously described (Figs. 155-1 and 155-2). When units of tissue are harvested solely with the perforating vessels leaving the entire muscle behind, they are termed “perforator flaps.” The flap is elevated suprafascial, beginning laterally. Perforating vessels from the deep inferior epigastric vessels are identified as they enter the flap (Fig. 155-3). These perforating vessels are then dissected toward the deep inferior epigastric vessels through their intramuscular course (Fig. 155-4). Once the vascular pedicle has been isolated, the flap is ready for transplant to the chest (Fig. 155-5). The deep inferior epigastric perforator (DIEP) flap allows for harvest of the lower abdominal skin and fat while leaving all of the rectus abdominis muscle in the abdomen (Figs. 155-6 and 155-7).
FIGURE 155-6
A. Intraoperative view of the lower abdominal skin raised superiorly before splitting the skin into two flaps, individually based on the deep inferior epigastric vessels. B. View of the conjoined DIEP flaps before division, while the vessels are still attached. Notice the opened abdominal wall fascia and rectus abdominis muscles. Bilateral isolated vascular pedicles are shown with the perforators entering the flap on each side. With this type of flap, no rectus muscle is removed.
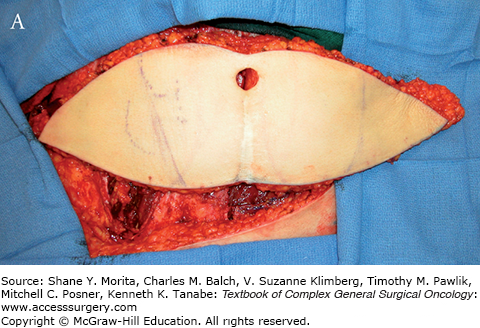
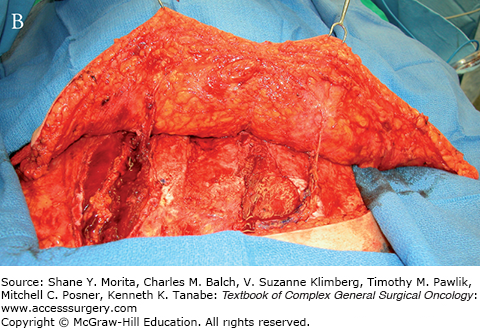
FIGURE 155-7
A. Intraoperative view of the DIEP flap isolated for a unilateral reconstruction. B. Intraoperative view of the corresponding abdominal donor site before primary repair.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree