Oberdorfer first described a tumor different from classic adenocarcinoma in the early 1900s, and referred to it as “karzinoid.” Historically, carcinoid has been the term to describe neuroendocrine tumors of the gastrointestinal (GI) tract. In reality, neuroendocrine tumors (NETs) are a heterogeneous group of tumors, including tumors originating from the stomach, pancreas, small bowel, rectum, appendix, and lung among other organs, and as a result there is a wide range of clinical courses. Because neuroendocrine cells secrete a variety of hormones and regulatory proteins, these tumors can secrete functional hormones. Carcinoid syndrome describes flushing, diarrhea, and cardiac valvular dysfunction associated with serotonin production, but tumors can produce a variety of hormones including gastrin, insulin, somatostatin, and glucagon. The GI tract and the pancreas are the most common site for NET formation, and they often metastasize to the liver through the portal vein. Because the primary tumors often have an indolent course, metastatic disease to the liver can be the primary presentation. This chapter focuses on the diagnosis and management of gastroenteropancreatic (GEP) neuroendocrine metastases to the liver.
Gastrointestinal and pancreatic NETs are the second most common malignancy of the digestive system.1 The incidence has increased markedly over the last 40 years.2 The incidence of all NETs including GEP tumors increased in the United States from an incidence ratio (IR) of 1 between 1973 and 1977 to IR of 3.65 between 2003 and 2007. In a population-based Canadian study, from 1994 to 2009, the incidence increased from 2.48 per 100,000 to 5.86 per 100,000, equivalent to a 2.36-fold increase.3 This has been shown to be secondary to increased detection.3 An estimated 70% to 80% of NETs are nonfunctional and peak incidence is 70 to 79 years old.4 Approximately 8.3% to 77% of patients with pancreatic NETs (pNETs)and 67% to 91% of patients with small intestine (or midgut) NETs develop liver metastases.5
There are few identified risk factors associated with GEP neuroendocrine malignancies. pNETs can be associated with Von-Hippel-Lindau (VHL) syndrome, multiple endocrine neoplasia 1 (MEN1), neurofibromatosis, and tuberous sclerosis. Upwards of 80% of patients with MEN1 have pNET and 10% to 20% of patients with neurofibromatosis and VHL syndrome. It is rare in tuberous sclerosis. Primary tumors of the pancreas and small bowel are most likely to develop liver metastases.
Pancreatic NETs have been studied extensively for molecular genetic alterations. The majority of pNETs occur sporadically; however, they also may be a part of MEN1, VHL syndrome, or neurofibromatosis. MEN1 demonstrates a defect on chromosome 11q13; MENIN, a 610 amino acid protein; VHL syndrome, a defect on chromosome 17q11.2; VHL, a 213 amino acid protein; and a gene on chromosome 17q11.2 neurofibromin, a 2485 amino acid protein. Malignant insulinomas demonstrate a high loss of heterozygosity (LOH) for markers on chromosome 22q (93%).6
Activating mutations in the ras family of proto-oncogenes (k-ras , h-ras, n-ras) are although absent or rare in large series of pNETs but have been reported as a frequent event in a study isolated to malignant insulinomas. However, the ras pathway may be involved in tumorigenesis of pNETs through promoter methylation of the RASSF1A gene which has been reported to occur at high frequency in pNETs. Comparing primary pNETs to their metastases show a more frequent genomic aberration in metastases when compared to their primary tumors.7 Investigation of multiple chromosomal markers revealed higher percentages of allelic imbalances in malignant pNETs and suggested that chromosomal instability may be a basis for malignant progression.8
Midgut NETs have an alternate profile, with accumulating evidence suggesting genes located on chromosome 18 . A genome-wide LOH screening showed 18q21 losses to be very frequent and specific in midgut NETs.61 These alterations were telomeric to the loci of colorectal cancer genes SMAD2, SMAD4, and DCC.
Although studies of hindgut NET molecular biology are relatively few, there have been reports of a high frequency of p53 gene abnormality with nuclear protein hyperexpression/accumulation in poorly differentiated NETs.9 In summary, the molecular genetic mechanism of the development of GI and pancreatic NETs remains complex and not well characterized. It has been established that the genes frequently seen in the development of GI and pancreatic adenocarcinomas are not commonly involved in GI and pancreatic NETs.
Neuroendocrine tumors often remain clinically silent and are diagnosed incidentally or after metastatic spread. Metastases to the liver often present nonspecifically with right upper quadrant pain, early satiety, jaundice, weight loss, and hepatomegaly. Functional NETs, most often arising from the jejunum, ileum, and cecum, can secrete active hormones, including serotonin, which leads to the carcinoid syndrome. The classic presentation of carcinoid syndrome is flushing, diarrhea, and right-sided heart disease.10 Carcinoid syndrome is more common with hepatic metastases as the liver can metabolize vasoactive substances secreted by functional tumors downstream, but once they are intrahepatic the substances disseminate directly into the systemic circulation. pNETs are less likely to be functional than GI tumors, but can secrete glucagon, somatostatin, insulin, and vasoactive intestinal peptide (VIP).10 Clinical manifestation of a glucagonoma includes necrolytic migratory erythema and diabetes mellitus, along with other nonspecific symptoms such as diarrhea, mental status changes, and weight loss. Insulinomas lead to hypoglycemia, and VIPomas have the classic presentation of WDHA (watery diarrhea, hypokalemia, and achlorhydria). Gastrinomas present with epigastric pain, reflux, and recurrent duodenal ulcers.
The World Health Organization (WHO) has developed the widely accepted classification scheme for NETs. It is clear that tumor differentiation is the most important prognostic factor for NETs, and WHO’s first set of guidelines in 2000 made a clear distinction between poorly and well-differentiated tumors.11 It was updated in 2010 and adapted the European Neuroendocrine Tumor Society (ENETS) grading system. It is based on mitotic count and Ki67. There are three classes: NET G1 (low grade tumor, mitotic count less than 2 per 10 high-powered fields (HPF) and/or Ki67 of less than 2%); NET G2 (mitotic count 2 to 20 per 10 HPF and 3% to 20% Ki67 index); and NEC G3 (mitotic count >20 per 10 HFP and/or Ki67 of >20%).12,13 Staging follows the American Joint Committee on Cancer (AJCC) and is specific to the site of primary tumor. Hepatic metastases are stage IV disease in all staging systems. Hepatic metastases may be classified as type I (single metastasis), type II (isolated metastatic bulk accompanied by smaller deposits), or type III (disseminated metastatic spread).14
Primary tumor location, class, and stage all have prognostic implications. For pancreatic primaries, stage IV tumors with metastatic disease have a 57% 5-year overall survival, compared to 92% for stage I disease.15 For midgut tumors, the overall survival is 100% for stage I and II tumors, 95% for stage III, and 73% for stage IV. Tumor grade is a more important prognostic factor, with 5-year overall survival for NEC G3 tumors being 7%, compared to 75% for NET G1 and 62% for NET G2.4 Tumors of the pancreas and small bowel have a worse prognosis than tumors of the GI tract (Fig. 129-1).
Liver metastases are a strong predictor of survival. For example, patients with gastrinoma can be expected to have a 95% 20-year survival that decreases to 15% with liver metastases.16 Similarly, patients with colon and rectal primary tumors have a 75% to 88% 5-year survival that decreases to less than 30% with liver metastases.5 Chromogranin A (CgA) is not a useful biomarker in diagnosis; however, it can be useful to assess the tumor burden and to follow for treatment response.17 Advanced age, male sex, low socioeconomic status, and rural living area are associated with worse survival.
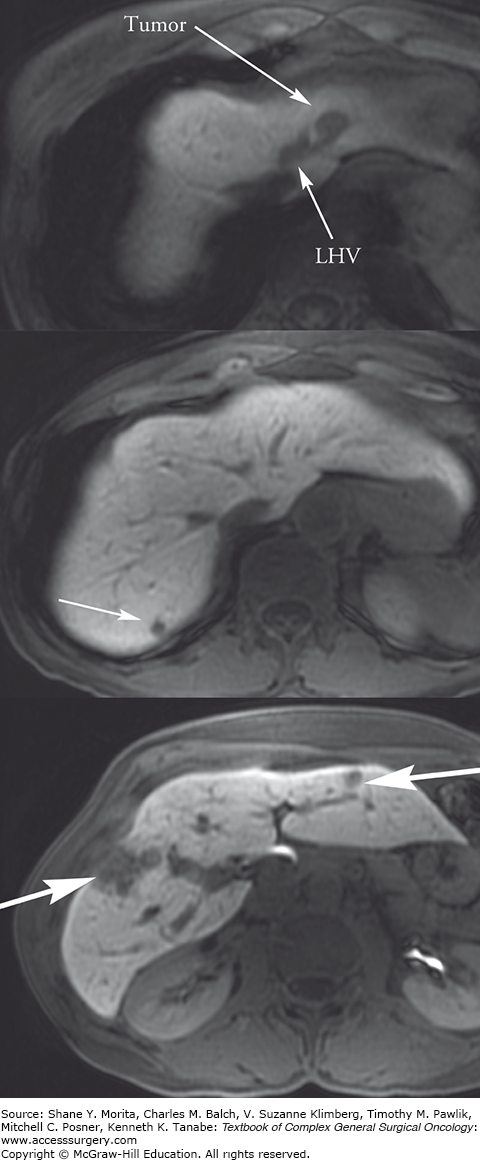
Initial diagnosis of the primary tumor and evaluation of metastatic disease are done through both standard abdominal imaging including computed tomography (CT) and magnetic resonance imaging (MRI), as well as ultrasound, endoscopy, endoscopic ultrasound, and capsule endoscopy. Somatostatin-receptor scintigraphy (SRS), more commonly known as an octreotide scan, has a 60% to 90% sensitivity for the diagnosis of GEP NETs and has therapeutic implications. New imaging modalities using conjugated radiopharmaceuticals including 68 -Ga dodecane-tetra-acetic acid (DOTA), 68-Ga DOTA octreotide (DOTATOC), 68-Ga DOTA octreotate (DOTATATE), 18F-flourinated levodopa (18F-dopa), 11C-5-hydroxytryptophan (11C-5-HTP), and other similar variations are more specific for localization, but not widely available.18,19 Positron emission technology (PET) is not routinely used in NETs because of low tracer uptake. However, the combination of 68-Ga DOTATOC and PET/CT identifies LM with a sensitivity of 82% to 100% and specificity of 67% to 100%.20,21 For management of metastatic disease to the liver, it is important to obtain detailed liver imaging for close attention to the number and location of the metastases as well as proximity to other structures, especially if resection or local ablative therapies are being considered (Fig. 129-2). Either triphasic CT or diffusion-weighted MRI is acceptable for imaging metastases; radiology centers should select the modality they use most frequently. There is some evidence that MRI may be more sensitive than CT in the detection of small metastases.22
FIGURE 129-2
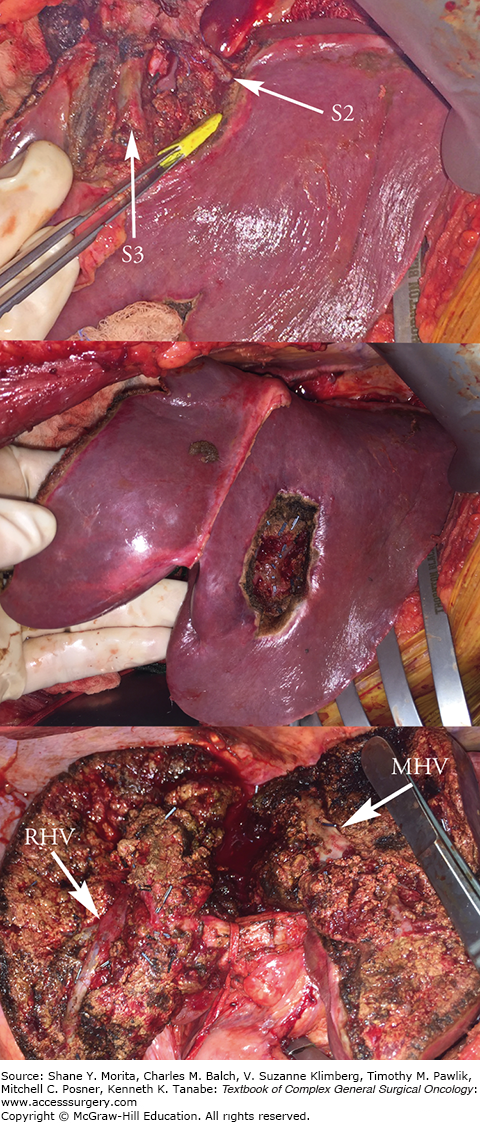
Resection of segment 2 lesion with sparing of segment 2 and 3 hepatic vein branches preserving outflow to the left lateral segment (top). Segment 3 lesion resected with clear margin notes the preservation of segments 2 and 3 (middle). Resection of anterior sector right lobe or segments 5 and 8 with right hepatic vein (RHV) and middle hepatic vein (MHV) as boundaries (bottom). Segment 7 lesions were resected nonanatomically. Parenchymal preservation techniques allow resection of diffuse disease with less risk than a corresponding extended procedure.
Systemic chemotherapy may be indicated for metastatic or rapidly progressive disease, although it is of limited utility. The most common chemotherapeutic agents used in NETs are alkylating agents including streptozocin and temozolide; however, they have significant renal and hematologic toxicity. They may be most effective in pancreatic primaries and in combination with fluorouracil (5-FU), where they have a high response rate and improved progression-free survival. In one study of patients with advanced pancreatic primaries, streptozocin plus 5-FU had a 33% complete response rate and 26-month median survival compared to 12% and 16.5 months in streptozocin alone.23 However, patients with metastases involving >75% of the liver had worse outcomes than those with <75% involvement.24 Similarly, temozolamide with capecitabine had a 70% response rate and progression-free survival of 18 months with good tolerance in patients with pancreatic primaries.25
Gastrointestinal or midgut NETs appear to be very chemoresistant. High-grade (NEC G3) cancers respond best to platinum-based therapy, with carboplatin plus etoposide and/or paclitaxel being the most common regimens, and may be more effective with a Ki67 of greater than 55%.26,27 Interferon alpha also may have some efficacy in both relief of symptoms and survival in patients with NEC G3 disease, but it has been limited by drug toxicity.24,28 A progressive randomized trial comparing interferon to lanetoide and combination therapy in 80 patients found low response rates for all arms, with only one patient each in the single therapy groups and two in the combination groups having a partial response. Fifty percent to 55% of patients in each arm had progression of disease, and side effects were more frequent with combination therapy.29
For less aggressive disease, everolimus and sunitinib are targeted therapies for advanced NETs. Everolimus is a mammalian target of rapamycin inhibitor, and is used alone or in addition to octreotide. The well-known RADIANT trials compared everolimus plus octreotide to placebo in pNETs. The second trial found a progression-free survival of 16.4 months compared to 11.4 in the control, with improved survival in pNETs. Radiant 3 looked at NET G1 and G2 pNETs and compared everolimus alone versus a placebo and also demonstrated an increase in progression-free survival.30,31 The use of everolimus in non-pNETs is less well studied. Sunitinib is a tyrosine kinase inhibitor that targets vascular endothelial growth factor (VEGF) and acts as an angiogenesis inhibitor. A phase III trial in low-to-intermediate grade pNETs showed a PFS of 11.4 months compared to 5.5 months for placebo.32 Pazopanib, a similar drug, has similar results in GEP NETs.33
Somatostatin analogues (SSAs) were initially used to treat symptoms in patients with functional tumors, but have some antiproliferative benefits in patients with octreotide avid disease.34 In a study of 33 patients with carcinoid syndrome, lanreotide was compared to octreotide with crossover at 1 month. Flushing and diarrhea improved equally in both groups (53.8% and 45.4% in lanreotide compared to 68% and 50% in octreotide); however, the patients preferred the easy delivery of lanreotide because of easier delivery.35 A randomized controlled trial in patients with metastatic midgut and pancreatic NETs (the CLARINET trial) demonstrated a significant increase in progression-free survival in patients with grade I and II tumors using lanreotide compared to placebo. Regardless of hepatic tumor burden36 there was an increase in progression-free survival in pancreatic primaries as well. SSAs appear to be indicated in patients with hormonally active midgut or pancreatic SSAs, and have efficacy in preventing disease progression in patients with metastases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree