INTRODUCTION
The ancient Greeks called it
phthisis, the Romans
tabes, and the Hindus
rajayakshma; in Victorian England, it was known as
consumption.
1 All of these names referred to the wasting illness that is characteristic of the disease we now call
tuberculosis (TB). Tuberculosis is a complex communicable disease of humans caused by the tubercle bacilli, a group of genetically related mycobacteria also known collectively as the
Mycobacterium tuberculosis complex. The tubercle bacilli are a group of slow-growing mycobacteria that include
M. tuberculosis,
M. africanum,
M. canettii,
M. bovis, and
M. microti. The first three members of this group are, at least to date, strictly human pathogens.
M. bovis causes illness in a variety of animals as well as in humans.
2,
3 and
4 Similarly,
M. microti generally causes disease in rodents, but has been linked retrospectively to infections in llamas, ferrets, and cats. It has also been implicated as the cause of pulmonary tuberculosis in a small number of humans.
5 With the advent of effective drug treatment in the 1950s and preventive therapy (or chemoprophylaxis, as it was then called) in the 1960s, many in the medical and public health communities, particularly in industrialized countries, assumed tuberculosis was conquered. This hubris led to several decades of neglect by the biomedical community, during which control efforts were ignored or deliberately weakened.
6 Unfortunately, the economic, social, and public health factors that foster the propagation of tuberculosis had not been eliminated, not even from the industrialized nations. Thus, in the 1980s and 1990s, as the deterioration of control programs coincided with the burgeoning epidemic of human immunodeficiency virus (HIV), tuberculosis rebounded. Numerous outbreaks were seen in the larger cities of the United States that had the highest incidence of HIV.
7 More serious, however, was the fact that in some areas of the developing world where tuberculosis and HIV are both endemic, the incidence of tuberculosis doubled, and healthcare facilities became overwhelmed by the dual epidemic.
6
The World Health Organization (WHO) declared tuberculosis to be a global emergency in 1993.
6 Eighteen years later, considerable progress has been made in managing this infection, but tuberculosis remains a leading cause of premature death in young adults around the world.
8 Roughly one third of the world’s inhabitants are latently infected with
M. tuberculosis. WHO has estimated that 8.8 million people developed tuberculosis and 1.45 million died from it in 2010, representing a significant decline from the 9.4 million cases reported in 2009.
8 Efforts to promote tuberculosis control have more than doubled since 1993, and the tuberculosis incidence rate has continued to decline annually since 2002 when it peaked at 142 cases per 100,000 population. Moreover, TB mortality has fallen by more than a third since 1990 and all WHO regions, other than the African Region, are on track to halve their 1990 mortality rates by 2015. Control efforts, highlighted by the DOTS (directly observed therapy, short course)/Stop TB Strategy, have saved almost 7 million lives by successfully treating 46 million of 55 million TB patients treated in DOTS programs.
8 The global success has been driven to a significant extent by China’s overwhelming achievements since 1990. That country has seen an annual 3.4% decline in its TB incidence, a halving of prevalence, and an 80% reduction in TB mortality.
8Despite this success, China and India account for one third of the world’s TB cases, with the primary drivers of the epidemic in these countries being social determinants such as tobacco exposure, diabetes, malnutrition, alcohol abuse, and indoor air pollution.
9 Meanwhile, in Africa, the high prevalence of
latent TB infection and HIV infection, a high prevalence of multidrug-resistant TB, and the complex epidemiology and natural history of tuberculosis continue to make control of the disease on this continent particularly challenging.
10
THE ORGANISM
The family Mycobacteriaceae, of the order Actinomycetales, is composed of a number of slow-growing, acid-fast bacilli. Most are saprophytes— useful inhabitants of soil and water that fix nitrogen and help degrade organic material. Some are pathogens in animals and occasionally cause opportunistic infection in humans.
11,
12 Only four species are highly pathogenic in humans:
Mycobacterium leprae (which causes leprosy) and three of the tubercle bacilli. The tubercle bacilli, or
Mycobacterium tuberculosis complex, comprise a group of five closely related mycobacteria that cause tuberculosis (
Table 18-1).
M. tuberculosis,
M. africanum, and
M. bovis are the most common cause of human tuberculosis, although
M. bovis is also known to cause disease in a variety of animal species. The other two members of the complex,
M. canettii and
M. microti, do cause tuberculosis in humans, albeit infrequently.
1,
3,
4 and
5,
12 In fact,
M. microti has been identified as a human pathogen only relatively recently.
5 Although they vary widely by favored host, phenotype, and pathogenicity, the
bacteria that make up the
M. tuberculosis complex share more than 90% of their genome and have identical 16S rRNA sequences.
2,
13 With the advent of the HIV epidemic, several other mycobacteria—most notably,
M. avium complex (MAC)— have emerged as common opportunistic pathogens and, in people infected with HIV, cause illness that is clinically similar to disseminated tuberculosis.
1,
13The bacteria that make up the
M. tuberculosis complex are slender, slightly curved, rod-shaped bacteria averaging 4 by 0.3 µm in size.
1,
3,
13 M. tuberculosis is strictly aerobic; in contrast,
M. bovis is microaerophilic and adapts more easily to nonpulmonary sites of infection. Like other mycobacteria, the tubercle bacilli have an unusual concentration of high-molecular-weight lipids in their cell wall, accounting for approximately 50% of their dry weight. This high lipid content makes these organisms hydrophobic and resistant to aqueous bactericidal agents and drying. It is also responsible for their acid-fast nature, a characteristic that is essentially synonymous with mycobacteria.
3,
13Mycobacteria are slow growing and fastidious in culture. Indeed, because
M. tuberculosis has a very long generation time (approximately 24 hours), culture is a slow process, often resulting in diagnostic delays and sometimes misdiagnosis.
5 Traditionally mycobacteria are grown on solid, enriched media, where colonies appear 4 to 6 weeks after inoculation. They can also be grown in liquid culture, where they form characteristic strings that can be seen by light microscopy. Rapid liquid culture systems (e.g., BACTEC) have been adapted for use with mycobacteria and allow identification of organisms in as little as 9-16 days, depending on the concentration of microbes in the specimen being tested.
3,
14 DNA probes speed speciation of organisms following growth. Alternatively, a number of biochemical tests can be used to speciate mycobacteria, though these approaches are time consuming.
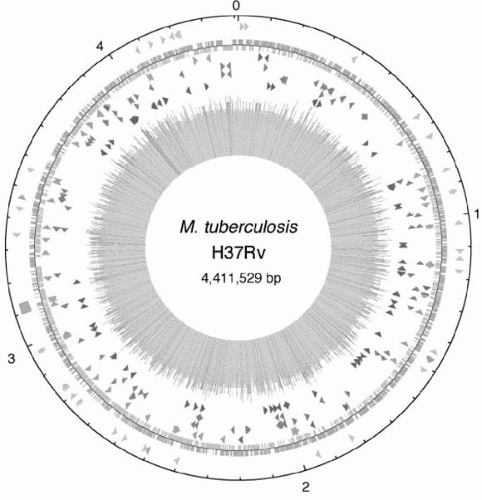
3,
15In 1998, a consortium of scientists deciphered and published the genome map of the H37Rv strain of
M. tuberculosis (
Figure 18-1). The complete genome was revealed to be 4,411,529 base pairs
long and contains approximately 4000 genes.
16 Since then, advances in genotyping technology have been rapidly applied to studies of the molecular epidemiology of the tubercle bacilli. Genotyping methods have been rapidly adopted, including restriction fragment-length polymorphism (RFLP), polymerase chain reaction (PCR)-based spoligotyping, and profiling of the mycobacterial interspersed repetitive units based on the number and size of the variable number tandem repeats in the genome (MIRU-VNTR). Most recently, the application of whole-genome sequencing analysis has been shown to be optimal, though this technique remains too costly for universal use. These methods vary in sensitivity and specificity, so some caution is needed when interpreting results.
18Genotyping studies are providing some interesting insights into the epidemiology of tuberculosis. For example, 15 years ago it was dogma that
M. tuberculosis was a mutated form of
M. bovis; the assumption being that around 7000-4000 BCE, when humans began domesticating animals, they were exposed to
M. bovis, which, over time, mutated into a human pathogen.
19 In a recent fingerprinting study, however, the genomes of 100 strains of
M. tuberculosis complex were mapped and compared. The genetic lineage developed from these analyses provides evidence that human
M. tuberculosis is not a mutation of
M. bovis; rather, both bacteria diverged from a common ancestor long before either infected humans
2,
13 (
Figure 18-2).
DNA fingerprinting is also being used with traditional field epidemiology to help link index and secondary cases and to distinguish active disease resulting from reactivation versus recent transmission. When distinguishing between reactivation and recent transmission, the presumption is that the genotype of cases due to reactivation will not match the genotype of other cases in the community because the infection was acquired at some distant point in the past. Conversely, in cases involving recent infection, the genotype of the organism should be shared with at least the index case and probably other cases in the community resulting from the same index case. Note, however, that the “orphan” isolates that do not share a genotype with any other organism from the community could still be related to other cases
in the community, but the link cannot be identified with traditional epidemiology; this can happen when the index case is sputum negative, or when the DNA fingerprint of the index is obtained for other reasons. Nevertheless, cluster analysis is fairly reliable and has provided new and interesting epidemiologic information about tuberculosis.
Before fingerprinting was commonly available, it was believed that most infections in immunosuppressed people in low-prevalence areas represented reactivation of latent infection. The populations at risk were often transient, and linking cases epidemiologically was difficult. More recently, DNA fingerprinting studies of community cohorts have identified some surprising clusters and helped guide the shoe-leather epidemiology needed to confirm the linkages suggested by fingerprint evidence.
20 Fingerprinting studies have also helped characterize the global distribution of genotypes, clarify patterns of transmission in communities, detect laboratory transmission, and prioritize control activities.
21,
22,
23,
24,
25 and
26 A U.S. study helped dispel the dogma that TB can be transmitted only through prolonged contact, and that casual transmission frequently occurs.
27 DNA fingerprinting is also being used to investigate the origins of susceptibility to the tuberculosis bacilli and innate host immunity to it.
18,
28,
29
CLINICAL MANIFESTATIONS
As discussed in more detail later, tuberculosis begins with latent infection that can progress to active disease. Latent tuberculosis infection (LTBI) causes no symptoms, and most latently infected people are unaware that they harbor tubercle bacilli. Tuberculosis disease generally affects the lungs and respiratory tract, but can strike nearly any organ system in the body. Both primary and reactivation tuberculosis disease can result in pulmonary or extrapulmonary manifestations. In immunocompetent people, approximately 80% of tuberculosis is pulmonary, whereas extrapulmonary disease is less common. Extrapulmonary disease is much more common in immunodeficient individuals and children.
34,
35 A small percentage of immunocompetent patients and many more immunodeficient ones develop both pulmonary and extrapulmonary tuberculosis.
35The onset of active tuberculosis is insidious, and the symptoms can be nonspecific. Pulmonary disease causes symptoms ranging from very mild to severe and can present with productive cough with or without bloody sputum, fatigue, anorexia, weight loss, fever, sweating and/or chills, and chest pain.
13,
34,
35 Extrapulmonary tuberculosis also causes fatigue and night sweats, but generally other symptoms specifically related to the affected organ system will be prominent.
13,
34,
35 and
36 The most frequent sites of extrapulmonary infection include the pleura, pericardium, larynx, lymph nodes, skeleton (particularly the spine), genitourinary tract, eyes, meninges, gastrointestinal tract, adrenal glands, and skin.
3,
37,
38 and
39 Systemic infection with tubercle bacilli occurs when hematogenous or lymphatic dissemination spreads the organism throughout the body, producing small nodules of infection in essentially every organ. Early researchers named disseminated disease
miliary tuberculosis because they thought the tiny nodules resembled grains of millet, particularly when seen by chest radiography.
13,
35,
40 Miliary tuberculosis is especially common in children and in people with immunosuppression.
7,
34,
41