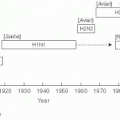
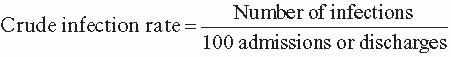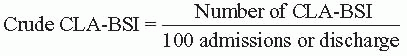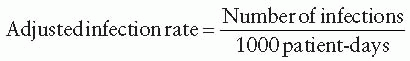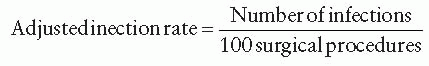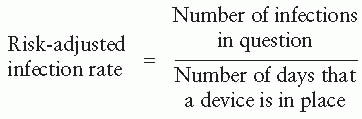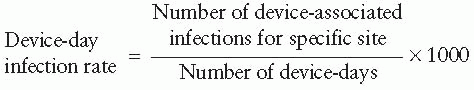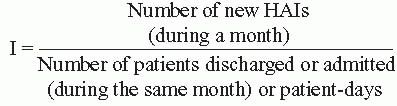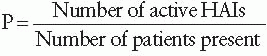Urinary Tract Infection
A urinary tract infection is an infection involving any part of the urinary system, including the urethra, bladder, ureters, and kidney. UTIs are the most common type of HAI reported to the NHSN, accounting for more than 30% of HAIs in acute care hospitals and perhaps even more in long-term care facilities.
4 Among UTIs acquired in the hospitals, at least 75% are associated with a urinary catheter. Approximately 15% to 25% of hospitalized patients receive urinary catheters during their hospital stay. Urinary catheterization not only causes bacteriuria that commonly leads to unnecessary antimicrobial use, but the urinary drainage systems also serve as reservoirs for multidrug-resistant bacteria and as a source of transmission to other patients. Prolonged use of the urinary catheter is the most important risk factor for developing a catheterassociated urinary tract infection. Given this risk, catheters should be inserted only when clinically indicated and should be removed as soon as they are no longer needed. Owing to this practice, it is the rate of UTIs associated with indwelling urinary catheters that is commonly reported to national databases such as NHSN.
1The pathogens most frequently associated with CA-UTI in hospitals reporting to NHSN between 2006 and 2007 were
Escherichia coli (21.4%) and
Candida spp. (21.0%), followed by Enterococcus spp. (14.9%),
P. aeruginosa (10.0%),
Klebsiella pneumoniae (7.7%), and
Enterobacter spp. (4.1%).
2 An increasing proportion of these organisms are becoming resistant to multiple antimicrobial agents.
174,
175 CA-UTIs have been shown to be associated with increased morbidity, mortality, hospital cost, and length of stay.
176,
177 and
178 Although morbidity and mortality from CA-UTIs are considered to be relatively low compared to other HAIs, such as central line-associated bloodstream infections and lower respiratory tract infections, the high prevalence of urinary catheter use leads to a large cumulative burden of infections. The CDC has estimated that as many as 139,000 hospital-onset, symptomatic CAUTIs occurred in 2007, resulting in as much as $131 million in excess direct medical costs.
179In view of the magnitude of this problem, in the United States, the Department of Health and Human Services (DHHS) developed an action plan to prevent HAIs, with a 5-year national prevention target of a 25% decrease from baseline for CA-UTI rates as measured by the NHSN. The CDC updated its guideline for prevention of CA-UTI in 2009 to put greater emphasis on the following aspects of care:
Appropriate urinary catheter use
Proper techniques for urinary catheter insertion
Proper techniques for urinary catheter maintenance
Quality improvement programs
Administrative infrastructure
Surveillance
An estimated 17% to 69% of CA-UTIs may be preventable with these infection control measures.
180 In fact, with the introduction of more CA-UTI prevention efforts and improved general infection prevention strategies in recent years, a pervasive and sustained decline in the incidence rates of symptomatic UTI and asymptomatic bacteriuria was observed in all major adult ICU types reported under the prior National Nosocomial Infections Surveillance (NNIS) and current NHSN system.
179,
180
Bloodstream Infections
Healthcare-associated bloodstream infections (BSIs) develop in more than 250,000 patients in U.S. hospitals every year, with an estimated attributable mortality of 12% to 25% for each infection.
181,
182,
183 and
184 The marginal cost to the healthcare system is approximately $25,000 per episode.
185 Most BSIs result from a secondary source such as a postoperative wound or intra-abdominal infection, urinary tract infection, or pneumonia. Primary bacteremia occurs when the source is unrecognized; most of these
cases are related to exposure to intravascular devices (IVDs).
184,
186 A vast variety of IVDs are employed in modern medicine. For example, a catheter can be designated by the type of vessel it occupies; its intended life span; its site of insertion; its pathway from skin to vessel; its physical length; its function (dialysis, pressure measurement); or some special character of the catheter, each of which is associated with a different infection risk.
183The terminology used to define intravascular catheter-related infections can be confusing. The terms “catheter-related bloodstream infection” (CR-BSI) and “central line-associated bloodstream infection” (CLA-BSI) are most widely used in the literature, sometimes interchangeably. In fact, CR-BSI is a clinical definition that requires specific laboratory testing (e.g., quantitative blood cultures of differential time to positivity) to help identify the catheter as the source of the BSI. In contrast, the term CLA-BSI is typically used for surveillance purposes and is defined as a primary BSI in a patient who had a central line within the 48-hour period before the development of the BSI and is not bloodstream related to an infection at another site.
1The most common organisms causing healthcare-associated BSIs are coagulase-negative staphylococci (CoNS; 31% of isolates),
Staphylococcus aureus (20%), enterococci (9%), and
Candida species (9%). CoNS,
Pseudomonas spp.,
Enterobacter spp.,
Serratia spp., and
Acinetobacter spp. are more likely to cause infections in patients in ICUs. Gram-negative bacilli accounted for one-fifth of CLA-BSIs reported to CDC and the Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE) database. The proportion of
S. aureus isolates with methicillin resistance increased from 22% in 1995 to 57% in 2001. Similar to the trend observed in CA-UTIs, the proportion of healthcare-associated BSIs due to multidrug-resistant organisms is increasing in U.S. hospitals.
187With the implementation of different prevention strategies in a variety of clinical settings, studies have consistently shown a declining incidence rate of CLA-BSI in the United States.
188,
189,
190 and
191 In 2011, the CDC updated its guideline for prevention of intravascular catheter-related infections to emphasize performance improvement by implementing bundled prevention strategies
183:
Educating and training healthcare personnel who insert and maintain catheters
Using maximal sterile barrier precautions during central venous catheter insertion
Using a greater than 0.5% strength chlorhexidine skin preparation with alcohol for antisepsis
Avoiding routine replacement of central venous catheters as a strategy to prevent infection
Using antiseptic/antibiotic-impregnated short-term central venous catheters and chlorhexidine-impregnated sponge dressings if the rate of infection is not decreasing despite adherence to the other strategies
The CDC guideline also emphasizes the importance of documenting and reporting rates of compliance with all components of the bundle as benchmarks for quality and performance improvement.
183 Since its publication, other, less expensive strategies such as chlorhexidine bathing have emerged as promising approaches.
192,
193,
194 and
195
Healthcare-Associated Pneumonia and Ventilator-Associated Pneumonia
Healthcare-associated pneumonia (HCAP) is the leading cause of death among hospitalized patients.
196,
197 Pneumonia is classified as HCAP when it occurs in any patient who (1) was hospitalized in an acute care hospital for 2 or more days within 90 days of the infection; (2) resides in a nursing home or long-term care facility; (3) received home intravenous antimicrobial therapy, chemotherapy, or home wound care within the past 30 days of the current infection; or (4) attended a hospital or hemodialysis clinic. HCAP may be further categorized as either early onset, in which pneumonia occurs during the first 4 days of hospitalization, and late onset, in which the disease occurs at least 4 days after admission.
1 HCAP typically increases the length of ICU stay from 5 to 7 days.
198Ventilator-associated pneumonia (VAP) arises in patients who have a device to assist or control respiration continuously through endotracheal tube or tracheostomy. This device must be present within the 48-hour period before the onset of infection.
1 A meta-analysis demonstrated that the incidence of VAP among patients receiving mechanical ventilation was 9.7% (95% CI: 7.0 -12.5) and incidence among patients in medical ICUs was 17.0% (95% CI: 5.9-28.0).
198 The additional cost for treating VAP was as high as $13,467 (in the year 2003).
198When microorganisms enter into and colonize the lower respiratory tract, HCAP can develop.
197 Under suitable circumstances, the pathogen can invade the mucosal and other host defenses, leading to disease.
197 Aspiration of oropharyngeal secretions
plays an important role for developing HCAP. Any conditions that precipitate, aggravate, or influence the volume or severity of aspiration can contribute higher risk for infection—for example, supine position, swallowing difficulty, gastric overextension, depressed sensorium, and enteral feeding.
197,
199,
200 Proton pump inhibitors (PPIs), which are commonly used among hospitalized patients for stress ulcer prophylaxis, may increase the risk of pneumonia.
201,
202,
203,
204,
205 and
206 One hypothesis suggests that this agent decreases gastric acidity, an innate aspect of immunity, and then produces bacterial overgrowth.
207 Oropharyngeal colonization with multidrug-resistant organisms (MDROs) increases risk of pulmonary infection with these organisms. Risk factors for infection with MDROs have been extensively studied and include the following:
Receiving antimicrobial treatment within the preceding 90 days
197,
208,
209,
210,
211 and
212
Current hospitalization of at least 5 days
Previous hospitalization in the preceding 90 days for at least 2 days
Immunosuppressive state
High frequency of antimicrobial resistance in the community or in the specific unit
The presence of risk factors for HCAP
Residence in a nursing home or extended care facility
Home infusion therapy (including antibiotics)
Chronic dialysis within 30 days
Home wound care
A family member with multidrug-resistant pathogen
197
Quasi-experimental studies have correlated the initiation of treatment with an inadequate spectrum of antimicrobial agent with increased mortality in HCAP/VAP patients.
213,
214,
215 and
216Intubation is the single most important risk factor: it increases the risk of developing pneumonia and VAP at least sixfold and increases mortality by 55%.
197,
217,
218 Once the first line of host defenses is breached by intubation and placement of an endotracheal tube, patients tend to aspirate organisms that colonize the oropharynx or upper gastrointestinal tract. Risk factors for developing VAP may be divided into those that can be modified and those that cannot be modified.
197 Nonmodifiable risk factors include advanced age, male gender, preexisting pulmonary disease, and severe underlying illness.
197,
219,
220 and
221 Modifiable risk factors include contaminated ventilator circuits, an endotracheal cuff with low pressure, multiple patient transfers, supine position of the patient, and gastric overextension.
197,
220,
221HCAP and VAP are mostly commonly caused by bacteria (multiple organisms), but can also be caused by fungi and viruses.
197 The most likely causative organism can be altered by the host’s immune status.
197 The most common pathogens causing HCAP are
S. aureus, Streptococcus pneumoniae, P. aeruginosa, and
Haemophilus influenzae.
208,
209,
211,
222,
223 The pathogens causing early-onset HCAP resemble those organisms associated with community-acquired pneumonia, such as
S. pneumoniae, Moraxella catarrhalis, and
H. influenzae. Similarly, gram-negative bacilli or
S. aureus, including MRSA, fungi, and
Legionella spp., occur in late-onset HCAP.
1 Worldwide, the pathogens causing VAP are predominately gram negative, consisting of
P. aeruginosa, Acinetobacter baumannii, and
Klebsiella spp.
224,
225,
226,
227,
228,
229,
230,
231,
232,
233 and
234 Compared with patients having HCAP, the prevalence of gram-negative pathogens is higher among VAP patients, while
S. aureus is more common in patients with HCAP.
235 Respiratory viruses can cause pneumonia at any time of hospitalization and are likely underappreciated as a cause of HCAP and VAP.
1 HCAP among immunocompromised hosts can be caused by inhalation of aerosols or droplets contaminated with
Legionella species,
Aspergillus species and other molds, respiratory syncytial virus (RSV), influenza virus, and other respiratory viruses.
236,
237,
238,
239,
240 and
241Outbreaks of HCAP/VAP have been documented in numerous healthcare settings. Organisms associated with these outbreaks include influenza,
242,
243,
244 and
245 RSV,
246,
247,
248 and
249 human metapneumovirus,
250,
251 adenovirus,
252 measles,
253 herpes simplex virus (HSV),
254 P. aeruginosa,255,
256,
257,
258 and
259 A. baummannii,260,
261 and
262 Enterobacter cloacae,263,
264 K. pneumoniae,265,
266 B. cepacia,267,
268 Serratia marcescens,269 M. catarrhalis,270 Neisseria meningitides,271,
272 Flavobacterium meningosepticum,273 S. aureus,257,
274,
275 and
276 S. pneumoniae,277,
278 Legionella spp.,
95,
97,
108,
115,
120,
279,
280,
281,
282,
283,
284,
285 and
286 and
Pneumocystis jerovecii.287,
288 and
289 Generally, in the setting of problems with ventilation or construction,
Aspergillus spp. and
Mycobacterium tuberculosis can also be associated with clusters in healthcare facilities (as discussed later in this chapter). The bacterial pathogens that cause HCAP or VAP may be multidrug resistant; in quasi-experimental studies, when an inadequate spectrum of antimicrobial agent was initiated as empiric therapy for HCAP/VAP, the mortality was increased.
213,
214,
215 and
216Many interventions to decrease HCAP/VAP have been investigated. In 2003, CDC published “Guidelines for Preventing Health Care-Associated Pneumonia.” These guidelines outline the importance of (1) environmental controls to prevented invasive pulmonary aspergillosis and Legionellosis,
(2) standard precautions, (3) immunizations, (4) measures to prevent aspiration, and (5) surveillance.
221In 2008, Society for Hospital Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA) published “Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals” in 2008.
290 To prevent VAP, these guidelines recommended interventions to decrease aspiration of secretions, including raising the head of bed, avoiding gastric overdistension, avoiding unintended extubation and reintubation, using a cuffed endotracheal tube with in-line or subglottic suctioning, and maintaining an endotracheal cuff pressure of at least 20 cm H
2O.
290 Furthermore, orotracheal intubation is preferable to nasotracheal intubation, as the latter may increase risk of sinusitis and VAP.
290 Three metaanalyses demonstrated the benefits of performing oral decontamination with antiseptic solution and favored chlorhexidine as the antiseptic of choice, although the impact was seen primarily in patients in cardiosurgical intensive care units.
291,
292 However, an impact on mortality has not been demonstrated from this intervention.
291,
293 It remains a standard of care to perform regular oral care with an antiseptic solution in mechanically ventilated patients.
290Surveillance for HCAP and VAP and process measures should be conducted in patients deemed at high risk for infection. The use of standard definitions is an important component of diagnosis of VAP, especially in ICU patients who have many risks for fever and pulmonary infiltrates. The VAP rate should be calculated by dividing the number of VAP cases by the number of ventilator-days.
Surgical Site Infection
Surgical site infection causes approximately onefourth of all HAIs, making it a major HAI especially among surgical patients. In the United States, SSI is the second or third most frequent HAI, responsible for 29% of all HAIs and 14% of all healthcare-associated adverse events.
180 As many as 5% of surgical patients develop SSIs.
294 This rate may be higher in patients cared for in developing countries. SSIs cause significant morbidity and account for 55% of all the extra hospital days attributed to HAIs.
295 Such infections increase length of hospital stay, readmission rates, and medical costs.
296,
297,
298,
299,
300,
301,
302,
303,
304 and
305 Patients with an SSI have a 2 to 11 times higher risk of death, compared to surgical patients without an SSI.
306 These numbers highlight the tremendous burden of these infections and the importance of preventing them. Because of the frequency, morbidity, mortality, and economic burden of these infections, SSIs are given the high priority for surveillance.
The definition of an SSI has been debated for years. For the purposes of surveillance, a reasonable definition should be highly sensitive but must balance specificity. It should be applied systematically and used to analyze rates, examine risk factors, develop prevention strategies, and assess trends over time. Ideally, the definition chosen should remain unchanged to facilitate comparisons of data through the years and to determine whether interventions implemented reduce these rates. In 1992, a consensus group that included CDC, SHEA, and the Surgical Infection Society (SIS) modified how SSI was defined and changed the name from “surgical wound infection” to “surgical site infection.”
307,
308 The definition for NHSN is currently being revised.
SSIs are divided into incisional and organ-space SSIs. Incisional SSIs are further classified as involving only the skin and subcutaneous tissue (superficial incisional SSIs) or involving deep soft tissues of the incision (deep incisional SSIs). Superficial infections require that at least one of the following occur within 30 days of the operation
309:
Pus appears from the incision
Organisms are isolated aseptically from cultured fluid or tissue
At least one of the following signs and symptoms: pain or tenderness, localized swelling, redness, or heat when the surgeon deliberately opens the surgical incision
The surgeon or attending physician diagnoses the infection
SSI secondary to prosthetic devices or foreign body can occur up to one year after the operation.
Only 33% to 67% of infected wounds are cultured, limiting the use of microbiologic case finding as a single case-finding strategy.
310 Direct inoculation is the most common pathway by which organisms cause infection. Endogenous flora can cause infections, especially those involving
S. aureus (including MRSA). Carriers of
S. aureus have a 2- to 14-fold increased risk of infection.
311,
312 Approximately 85% of
S. aureus infections are attributable to a carrier state, a fact that has led to the development of decolonization strategies including the use of intranasal mupirocin and chlorhexidine bathing.
312,
313 Additionally, SSIs may result from exogenous sources—for example, a carrier healthcare worker (e.g.,
S. aureus and group A streptococci) contaminated equipment, and a defect in the ventilation system of the operative theater.
149,
314,
315,
316 and
317 In those cases, the most common causes of SSI are
S. aureus, enterococci, and coagulase-negative staphylococci. However, the infecting organisms vary according to the site of the surgical
incision.
309,
318 For operations that involve only skin or a clean wound, the most common pathogens are the skin flora (e.g.,
S. aureus, coagulase-negative staphylococci), while gram-negative and anaerobic organisms are often found in patients who undergo operations that involve the bowel, such as colon surgery.
309 Table 14-4 shows the classification of surgical site infections.
Outbreaks of SSIs are rarely associated with HCPs (
Table 14-5). For example,
S. pyogenes (group A streptococci) is occasionally reported to cause SSIs, most commonly spread from asymptomatic individuals including HCPs.
149,
150,
151,
152,
153,
154,
155,
156,
157 and
158,
320,
321 In this case a single case should prompt increased surveillance and some investigation. Similarly, HCPs can be carriers of
S. aureus and an investigation of HCP is indicated when fingerprinting of strains reveals they are clonal and suggests a single source. Outbreaks of
P. aeruginosa have been caused by surgical staffs with onychomycosis.
144,
147Risk factors that increase a patient’s risk of developing an SSI can be categorized as intrinsic (patient related) or extrinsic (operation-related).
306 The patient’s risk may increase if he or she has more than one risk factor. Patient characteristics, including the underlying medical condition(s) and the severity of the underlying disease, can increase the risk of developing an SSI. To date, very few controlled studies have examined the relative importance of these risk factors. Those additional risk factors that definitely increase the risk of developing an SSI include morbid obesity, smoking, old age, diabetes mellitus, a prolonged preoperative hospital stay, or a preoperative infection.
83,
306,
309,
329,
330 and
331 Several studies have shown that patients who are malnourished, have low albumin, have cancer, or are receiving immunosuppressive therapy are at increased risk of developing an SSI.
306,
332,
333 and
334 Unfortunately, it is frequently difficult to ameliorate these risks. If the patient’s surgery is not urgent, and the health condition, such as diabetes, can be controlled or the patient surgery can be delayed, then a delay until the patient’s health improves can improve his or her outcome.
Certain practices and procedures that occur in the operating room or during the perioperative period can increase the risk of SSI. In some cases, HCPs can alter their practices and potentially the patient’s outcome. Those surgical and environmental factors that may
alter the patient’s risk of developing an SSI include a wound classified as contaminated or dirty (
Table 14-4), a surgical procedure involving the abdomen, a long operative time, hair removal by razor (especially the night before surgery, so that preoperative colonization occurs), multiple surgical procedures, poor hemostasis during the procedure with blood loss and the need for transfusions, prolonged presence of drains, a lessskilled or inexperienced surgeon, and a low intraoperative body temperature.
335,
336,
337,
338,
339,
340 and
341 Risk factors for SSIs are summarized in
Table 14-6.
One of the most important interventions—if not the most important single intervention—is the appropriate use and timing of perioperative antimicrobial prophylaxis.
353 Pathogens that infect surgical sites during surgical procedures can be acquired from the patient, the hospital environment, or HCPs. The patient’s endogenous flora are responsible for most infections. In surgical procedures involving the gastrointestinal, respiratory, genital, and urinary tracts, antimicrobial prophylaxis has proved efficacious in reducing SSIs. For many cardiac, neurosurgical, and orthopedic procedures, prophylaxis decreases the risk of developing an SSI. For most clean surgical procedures, antimicrobial prophylaxis remains controversial, although experts suggest giving patients a prophylactic antimicrobial agent when a foreignbody implant is inserted or if an infection occurring in a clean procedure would be associated with severe or life-threatening consequences (e.g., valve replacement).
306 Finally, if the perioperative antimicrobial agent is administered after the incision, the risk of an SSI increases sixfold above the risk when it is administered 30 minutes to 1 hour (2 hours is allowed
for vancomycin and fluoroquinolones) before the incision.
306 Antimicrobial prophylaxis should be stopped within 24 hours after the surgery for most procedures.
306The patient’s skin is a major source of intraoperative contamination and organisms. Resident flora such as coagulase-negative staphylococci can be found in the deeper layers of the dermis; hence, cleaning and disinfecting skin before operation by antiseptic reduces the risk of SSIs. A recent randomized study demonstrated the benefit of using a chlorhexidine-alcohol based preparation for skin antisepsis over povidone-iodine in patients undergoing clean-contaminated operations.
354Preoperative cleaning of the operative team’s hands is equally important and is performed by the operative team. Recent studies have demonstrated that the timeconsuming hand scrubbing with brushes for 10 minutes did not decrease SSI rate and that a shorter scrub or hand rubbing by aqueous alcohol solutions is adequate.
355 Scrubbing with brushes may damage the HCP’s skin and result in increased shedding of bacteria.
356The operating facilities should be designed to promote best cleaning and disinfection practices, provide appropriate air handling, and assure that HCPs can follow best practices. The operating rooms should have a semi-sterile and sterile core, and the operating room itself must have higher pressure than the outside corridor (positive pressure). The doors to the operating room suite should remain closed at all times and the traffic in the room should be minimized.
357 Pass-throughs should be designed to minimize the traffic even further. Most exogenous wound contamination occurs during the operation through contact or airborne transmission of organisms; hence, events occurring after the operation (e.g., ward dressings and isolation techniques) are less likely to contribute to SSIs.
Finally, the importance of transparency and sharing SSI rates with surgeons, operating room staff, and leadership cannot be overemphasized. Reporting of surgeon-specific SSI rates can also have a role, although their use for credentialing purposes should be carefully considered. Programs that perform surveillance for SSIs may reduce these rates as much as 35% by reporting the rates associated with specific surgical teams.
308 To save resources, some programs have adopted methods that group the entire surgical population into patients who are at a higher or lower risk of developing SSIs. In risk stratification, procedures are classified and reported using a risk index. The NNIS risk index—the most commonly used stratification process—groups procedures into three categories.
358One of the major challenges in infection prevention and control is deciding when to include postdischarge surveillance for detecting SSIs. This strategy is important, as these infections can develop after the patient has been discharged home. Routine surveillance processes may not capture such SSIs (
Table 14-7).
310,
358,
359,
360,
361,
362 and
363 Thus surveying surgeons’ offices to monitor each patient for a
defined period after his or her surgery should be incorporated into surveillance strategies. Telephone surveys and questionnaires sent to patients and/or physicians have been used as well. Which method provides the most accurate data has not yet been determined.
308 While more difficult to perform, surveys that cover the postdischarge time period are important, as those SSIs that develop after either clean or clean-contaminated procedures are the most likely to occur after hospital discharge.