Epidemiology of Helminth Infections
Clive J. Shiff
INTRODUCTION
Parasitism is a way of life. Over evolutionary time, the niche by which one species depends on another for subsistence has been elaborated in countless ways. Not only have parasitic species developed a means to adapt to existence in the gut, in the tissues, and within the cells of their hosts, but these species have also evolved mechanisms to distribute their progeny so that they can readily find and be taken up by a new host. Parasites have adopted a variety of forms, some of which may appear grotesque. They have evolved stratagems to evade the immune defenses of their hosts, and they have coevolved with their hosts to the extent that they adapt to the behavior patterns of normal life and exploit these so as to migrate to other sources of hosts. All of these factors influence in some way the epidemiology of parasitic infections.
Transmission and acquisition of parasites by any naive host involves three factors: (1) a source of infection or reservoir must be present from which the parental generation of the parasite radiates; (2) a means of transmission must exist by which the parasite gains access to the host; and (3) a susceptible host must be available. As these factors bear directly on the severity of parasitic infections and their importance in the communities of humans, they must be considered in any study on the epidemiology, health impact, and control of the infection. Parasites have evolved numerous strategies to be successful within their various hosts, but transmission essentially involves two types of cycle. The first is the direct cycle, where transmission is from person to person, usually through fecal waste in the environment. The second is the indirect cycle, which involves additional hosts or vectors that actively transfer the parasite from one host to another.
To demonstrate the complexity of this process, one example of each cycle will be discussed in this chapter in detail. The direct cycle will be explained through consideration of the life cycle and epidemiology of hookworms. The indirect cycle will consider the complex epidemiology of schistosomes. The hookworms affecting humans belong to two species of nematode parasites, which produce similar infections, but because they are difficult to differentiate clinically and epidemiologically, they usually are considered together. Transmission of these parasites depends on fecal contamination of the environment, absence of acceptable latrines, and a barefoot lifestyle. The schistosome parasite is a blood-dwelling trematode that has a complex life cycle involving living in freshwater molluscs as well as in the bloodstream of its definitive host. This parasite has coevolved in tandem with its aquatic and human hosts, producing a well-balanced association. However, the recent settling of human populations in new areas, with their need for water and their changing agricultural activities, as well as the burgeoning of these human populations, has increased the transmission of the parasite and, therefore, has produced severe and debilitating infections. Indeed, overt disease caused by these parasites often results when the ecological balances to which the various populations have been adapted become unstable or break down, resulting in increasingly severe levels of infection.
HOOKWORM PARASITES OF HUMANS
Hookworms belong to the phylum Nematoda. Nematodes are tubular animals, diecious, with a definite body cavity in which the various organs are suspended. Their gut is tubular, commencing in a
complex oral region where the mouth and pharynx may have cutting teeth or plates, and the pharynx may be adapted for sucking and ingesting food. A hookworm’s body is covered with an outer cuticle, which is a complex structure consisting of several layers that serves as a protective cover for the worm. These parasites are equipped only with longitudinal muscles, which accounts for the sinuous movements characteristic of the group.
complex oral region where the mouth and pharynx may have cutting teeth or plates, and the pharynx may be adapted for sucking and ingesting food. A hookworm’s body is covered with an outer cuticle, which is a complex structure consisting of several layers that serves as a protective cover for the worm. These parasites are equipped only with longitudinal muscles, which accounts for the sinuous movements characteristic of the group.
In parasitic nematodes, the female is usually larger and packed with large ovaries and uterus. The males may exhibit complex external copulatory structures, which are characteristic of some species and which are used in identification. Eggs, which are usually characteristic for each species, are laid in large numbers; they may be embryonated or contain developing larvae. Usually the larval development takes several days, after which a first-stage larva emerges. This stage is able to ingest food and will proceed through two molts to reach the L3 stage, which in hookworms is infective; at this point larvae can no longer ingest food. The infective L3 larva (called the filariform stage) is able to attach to and penetrate the skin of the next host by using proteolytic enzymes secreted in the apical area of the worm. Altogether, four larval molts occur before the adult develops, and it is always the third-stage larva that is infectious.
Life Cycle
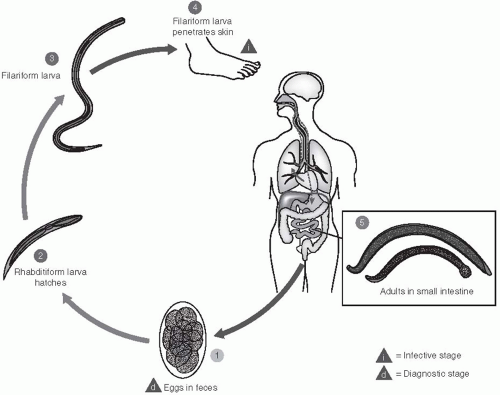
Two species of hookworm are known to infect humans: Ancylostoma duodenale and Necator americanus. The life cycles of these two parasites are similar and, therefore, will be discussed together (Figure 28-1). Embryonated eggs are passed in the feces of an infected person. In a suitable environment—one that is shady or dark, moist, and warm (22°C to 32°C)—the eggs will hatch within 24 to 48 hours, releasing first-stage larvae. These forms are not infective; they can ingest food, and will soon molt into second-stage larvae after approximately 3 days. A second molt occurs after about 6 days, and the resulting third-stage larva is infectious—at the filariform stage. These larvae are unable to feed; however, under ideal conditions, they can survive and remain infectious for several weeks.
Larvae invade by penetrating the skin between the toes or through the feet or ankles. However, they can also be transmitted through eating or handling unwashed, contaminated vegetables. Evidence also suggests that A. duodenale can be transmitted to suckling infants through breastmilk. The filariform larvae secrete proteolytic enzymes that facilitate the penetration through the skin; they then enter the blood circulation and usually molt once more as they pass through the lungs. From the alveolar spaces, the larvae are coughed up in sputum, are swallowed, and thus gain entry to the human gut. The worms then reach the intestine where, as adults, they mate. They adhere to and lacerate the intestinal mucosa with their strong oral plates or teeth, they pump blood into the gut by means of the powerful muscular pharynx, and they can continue to flush their gut with a stream of blood from the intestinal vessels they have penetrated. Thus, apart from consuming blood as a source of food, the parasites cause considerable amounts of blood to be lost and voided in the feces of the host. Egg production commences 4 to 8 weeks after the initial infection and the worms can live approximately 3 years.
Epidemiology of Hookworm Infections
Hookworm infection is a worldwide problem that is most prevalent in warm, humid areas or environments. It is widespread in the tropics, but is also common in warm, wet areas of the temperate zones; it is frequently associated with anemia in the affected populations.
Vulnerable populations include children, pregnant and lactating women, and women who menstruate heavily. The prevalence of geohelminth infections is age related, possibly because of immunologic factors or specific activities or behavior patterns related to age. The prevalence of hookworm is found to be lower among children younger than 5 years, but gradually increases with age; by age 8 years, a marked increase in prevalence occurs, which diminishes in later life. A clustering of infections is seen in certain children: evidence shows that heavily or lightly infected children have a statistical predisposition to acquire similar infection intensities following deworming procedures if patterns of exposure have not changed.
The highest prevalence of hookworm infection is found in males, teenagers, and young adults, which may be related to occupational hazards. For example, tending crops such as in rice paddies, where one must stand in the fields for many hours, increases the exposure to hookworm and the likelihood of becoming infected. Other risk factors are associated with the extent of outdoor defecation, presence of defecation fields, and the type of soil, which should be loose and hold moisture well, thereby providing a refuge for the infective larvae. Poor standards of living and sanitation are the major determinants of hookworm prevalence. Infection rates are usually higher in rural areas than in urban areas, and higher in
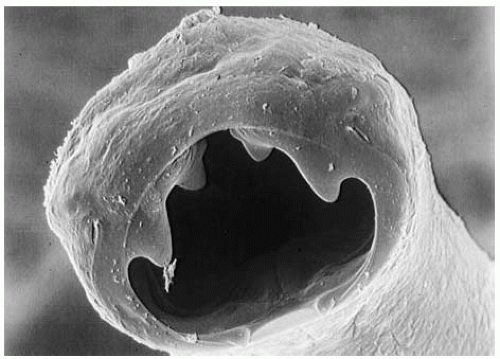
people who rank low on the socioeconomic scale. In parts of Europe and North America where hookworm was previously common, the infections have been all but eradicated because of improved access to effective sanitation and improved living conditions, including the wearing of shoes. Figure 28-2 shows a photograph of Ancylostoma duodenale.
people who rank low on the socioeconomic scale. In parts of Europe and North America where hookworm was previously common, the infections have been all but eradicated because of improved access to effective sanitation and improved living conditions, including the wearing of shoes. Figure 28-2 shows a photograph of Ancylostoma duodenale.
Control Measures
No prophylactic drugs are available for hookworm infections, although iron therapy is useful in preventing serious nutritional depletion, especially in women and children. Mebendazole is often used to treat both hookworm and other geohelminth infections. Mass chemotherapy is effective in reducing the prevalence of all geohelminths, but its effects are short-lived if no improvements are made in sanitation and health education.
Considerable lack of knowledge exists about the transmission of intestinal parasites in many parts of the world. A study of mothers in urban slums in Sri Lanka demonstrated that 42% of mothers thought that parasites were acquired through eating
sweets, 25% thought that they were transmitted through other children, and 25% did not know how the parasites were acquired. None of the study participants were aware of the relationship between contamination of soil with feces and transmission of the worms.1 Kendall et al.2 also mention that some societies consider that worms are normal symbiotes of the gut, which when mistreated (e.g., during a period of starvation or low food intake) can cause illness such as diarrhea.
sweets, 25% thought that they were transmitted through other children, and 25% did not know how the parasites were acquired. None of the study participants were aware of the relationship between contamination of soil with feces and transmission of the worms.1 Kendall et al.2 also mention that some societies consider that worms are normal symbiotes of the gut, which when mistreated (e.g., during a period of starvation or low food intake) can cause illness such as diarrhea.
One of the best measures an individual can take to avoid hookworm infection is wearing adequate footwear. This practice reduces the risk of infection, especially if shoes are worn in latrines, in the vicinity of human habitation, and during agricultural work. The appropriate use and maintenance of latrines also makes a difference. Hookworm eggs and larvae do not survive more than 1 or 2 months in soil, even under ideal conditions. Use of latrines helps reduce contamination of soil with the parasite and, therefore, reduces transmission. Additionally, minimizing the use of night soil as a fertilizer helps prevent the contamination of vegetables with parasites. Measures that can be used to prevent the transmission of all geohelminths include cleaning up stools of infants too young to use latrines; maintaining personal hygiene and care in preparing food, especially vegetables; the use of adequate footwear; and public education in elementary sanitation.
Potential for Vaccines?
There is much similarity between hookworm parasites of dogs and humans not only in terms of parasite morphology, but also in terms of the host response; therefore it is appropriate to study the immune responses in dogs and extrapolate the findings to humans. This subject is well reviewed by McSoreley and Loukas.3 In experimental conditions, strong antibody responses representing all classes of immunoglobulins are noted, with parasite-specific immunoglobulin M (IgM) becoming detectable 6 weeks post infection, and immunoglobulin G (IgG) increasingly being detectable 8 weeks after infection. Immunoglobulin E (IgE) response develops slowly after multiple infections and appears to afford protection to further infection. Cytokine responses are complex and induce polarized TH2 responses, although this relationship is not clear in all instances. Finally, in the immune repertoire, cellular responses have been noted, again with much complexity.
Much of the work investigating the human response to hookworm infection has been done in the search for a hookworm vaccine. In fact, irradiated L3-stage larvae of Ancylostoma caninum have been used as a vaccine in dogs. Although this vaccine achieved high levels of protection, its use was discontinued because it did not achieve sterilizing immunity and was expensive to produce.4 The approach to hookworm vaccination in humans is directed against the invasive larval stage of Necator americanus termed NA-ASP-2, and the resulting product has been through safety trials in both the United States and Brazil. However, the efficacy and production of such a vaccine have not yet been tested.5
SCHISTOSOME PARASITES IN HUMANS
Three important species of schistosomes infect humans, with an additional two that occur in restricted areas.
Schistosoma haematobium occurs primarily in Africa, with extensions into the Middle East and western Asia. This species lives in the vesicular veins and capillaries of the bladder mucosa and causes the condition known as urinary schistosomiasis (bilharzia).
Schistosoma mansoni occurs primarily in Africa, but is also found in the northern parts of South America (Brazil) and the Caribbean. Adults of this species normally live in the capillaries that drain the mesenteries; also sometimes found in the liver sinuses, they can produce the condition called intestinal schistosomiasis (also known as intestinal bilharzia).
Schistosoma japonicum occurs mainly in China and parts of Southeast Asia, particularly in the Philippines. This species, which occupies the same region of the body as S. mansoni, is a more virulent form of the parasite, and it frequently produces severe sequelae.
The other human-infective species are S. meekongi, which is related to S. japonicum, found in Vietnam and adjoining territories, and S. intercalatum, which is related to S. haematobium, found in Cameroon and parts of West Africa.
Because of their reliance on freshwater snails as aquatic intermediate hosts, the entire distribution of these parasites is associated with water resources in which the appropriate snail species are found.
Life Cycle
Adult schistosomes differ from typical trematode worms in their narrow, elongated shape and separate sexes. The male is the larger of the two sexes, approximately 1.0 to 1.5 cm in length, with a cylindrical body folded to form a ventral gynecophoric canal in which the longer, slender female is embraced for most of the time. Both worms have two suckers—an oral sucker surrounding the mouth and a ventral sucker
or acetabulum. The mouth leads into a blind gut that bifurcates along most of the length of the body. In the female, this area is dark with hematin derived from the digestion of blood cells. The number of testes in the male, the length of the uterus, and the shape of the eggs in females are distinctive to the species.
or acetabulum. The mouth leads into a blind gut that bifurcates along most of the length of the body. In the female, this area is dark with hematin derived from the digestion of blood cells. The number of testes in the male, the length of the uterus, and the shape of the eggs in females are distinctive to the species.
Eggs are deposited in the fine capillaries of the organ in which the worms are living. In vesicular schistosomiasis, this is the bladder; in the intestinal form, it is the intestinal mucosa. The eggs break through into the lumen of the bladder or gut, usually with a small amount of bleeding, and are passed to the exterior in the urine or feces. Many eggs do not break through the mucosa and remain in the tissue or are flushed into the liver, where they form a nidus for granulomata to develop. In severe infections, these granulomata damage the affected organ. In some cases of ectopic egg deposition, severe longterm paraplegia can occur when the base of the spinal cord is involved.
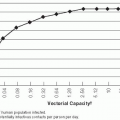
If the schistosome eggs are deposited in freshwater, or in a place where they can be soon washed into the water, the cycle continues. The eggs hatch, and a free-living, ciliated form, known as the miracidium, emerges. These miracidia use a number of environmental cues to seek out appropriate intermediate host snails. Miracidia move quite rapidly and can cover great distances in their search for snails. In some recent studies in Egypt, S. mansoni miracidia were shown commonly to infect snails 5 to 6 m distant, and some infected snails more than 20 m distant. The association is specific, so only the correct species of mollusc will sustain the infection. The miracidia do not ingest food and so have an infective life limited to approximately 5 to 6 hours. During this time, they must find the appropriate snail. The miracidium attaches to the snail and secretes proteolytic enzymes that penetrate into the tissues of the snail. The parasite then commences a process of asexual development. The miracidium enlarges into a mother sporocyst, a saclike organism that later buds off additional daughter sporocysts from layers of germinal epithelium. These daughter sporocysts migrate to the digestive gland of the snail, where they grow and finally produce copious numbers of the next larval stage, the cercaria.
Cercariae emerge from infected snails in response to sunlight after a prepatent period of approximately 30 days, although this could be much longer in cool weather. Cercariae normally emerge around midmorning, and continue emerging from the snails throughout the day, although by afternoon the numbers soon decline. The cercariae are furcocercous (Figure 28-3) and move by vibrating their forked tail. Their main movement is up and down—that is, vertical rather than horizontal—and their target is the skin of a nearby human being. They respond to appropriate skin lipids that stimulate the process of penetration, a process that must occur within 6 to 12 hours after emergence, as cercariae, too, have no means to ingest nutrients.
When they commence penetration, cercariae secrete proteolytic enzymes, bore through the skin of the victim, then shed the tail, and, by contortions, penetrate into the subdermis, invade the lymphatic system, and move via the circulatory system to the lungs. In the lungs, the developing form, known as the schistosomulum, remains for a few days before continuing in the circulatory system to the liver. In the liver, the schistosomula mature and move to the end organ system in pairs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree